-
- 15383190639
- admin@86-ss.com
Your Location:Home >Products >Organic chemicals >61177-45-5

Product Details
|
Cautions |
Before taking a combination of potassium clavulanate with any other penicillin-based antibiotic, one should discuss with medical providers and let them know whether they have severe kidney disease, jaundice or liver problems. In addition, it is important to inform the physician if an individual is allergic to penicillin or cephalosporin antibiotic, such as Amoxil, Levaquin, omnicef, and cefzil. Although it is not clear with this drug may harm an unborn baby, it is important to inform the physician if one is pregnant or intending to become pregnant. In addition, studies have indicated that pass into breast milk, thus may affect a nursing infant. As such, it is important to inform the doctor if one is breast-feeding. Children should never be given potassium clavulanate without consulting a physician. |
|
Administration |
The drug should be take according to the prescription given by the doctor. Potassium clavulanate is taken orally every 12 hours at the start of a meal to minimize side effects such as stomach upset. The extended release of the potassium clavulanate tablet must never been chewed or crushed. The medicine should be swallowed as a whole while the chewable tablet must be chewed before swallowing it. Before measuring a dose, the liquid medicine must be shaken well. The medicine should be only used for the full length of prescribed time. It is important not to skip any doses as it may lead to further infection that is resistant to antibiotics. The medicine should never be taken with or just after ingesting a high-fat meal as it will make it harder for the body to absorb the drug. |
|
Side Effects |
Potassium clavulanate may cause diarrhea that is bloody or watery when taken in combination with amoxicillin. It may also lead to severe stomach pain, swelling of the face or tongue, loss of appetite, fever, dark color urine, weakness, or confusion. The medicine may cause jaundice or yellowing of skin as well as vaginal itching or discharge. |
|
Manufacturing Process |
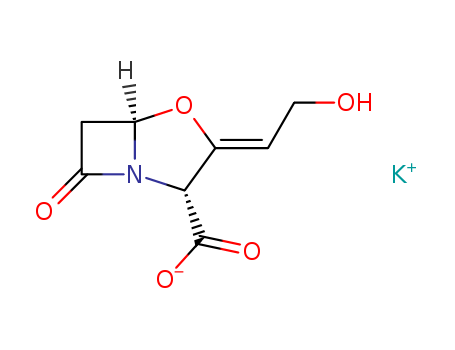
Clavulanic acid may be obtained by aerobic cultivation of Streptomyces clavuligerus in conventional nutrient media at, for example, about 25°-30°C under roughly neutral conditions. Cultivation of Streptomyces clavuligeru: Streptomyces clavuligerus was cultivated at 26°C on agar slopes containing 1% Yeatex (yeast extract) ("Yeatex" is a Registered Trade mark), 1% glucose and 2% Oxoid agar No. 3, pH 6.8. A sterile loop was used to transfer mycelium and spores from the slope into 100 ml of a liquid medium in a 500 ml Ehrlenmeyer flask. The liquid medium had the following composition: Oxoid Malt Extract 10g/L, Oxoid Bacteriological Peptone 10g/L, Glycerol 20 g/L, Tap water 1 L. The medium was adjusted to pH 7.0 with sodium hydroxide solution and 100 ml volumes dispensed into flasks which were closed with foam plugs prior to autoclaving at 15 lb/sq.in. for 20 min. An inoculated seed flask was shaken for 3 days at 26°C on a rotary shaker with a 2 inch throw and a speed of 240 r.p.m. Production stage flasks containing the liquid medium described above were inoculated with 5% vegetative inoculum and grown under the same conditions as the seed flask. Clavulanic acid may be extracted from the culture medium. Normally the cells of the Streptomyces clavuligerus are first removed from culture medium by filtration or centrifugation. Then clavulanic acid is extracted into an organic solvent, for example, n-butanol or ethyl acetate, or n-butyl acetate, or methyl isobutyl ketone. Then n-butanol fraction are treated with new aqueous phase using potassium hydrogen carbonate and then this aqueous phase is washed with n-butanol. This aqueous extract, after separation of the phases, is concentrated under reduced pressure. Freeze-drying at -20°C may also beemployed to provide a solid crude preparation of the potassium Z-(2R,5R)-3- (β-hydroxyethylidene)-7-oxo-4-oxa-1-azabicyclo[3,2,0]heptane-2-carboxylate (clavulanate potassium). |
|
Therapeutic Function |
Beta-lactamase inhibitor |
|
Definition |
ChEBI: A potassium salt having clavulanate as the counterion. It acts as a suicide inhibitor of bacterial beta-lactamase enzymes and has only weak anitbiotic activity when administered alone. However it can be used in combination with amoxicillin trihydrate (under the trade name Augmentin) for treatment of a variety of bacterial infections, where it prevents antibiotic inactivation by microbial lactamases. |
InChI:InChI=1/C8H9NO5.K/c10-2-1-4-7(8(12)13)9-5(11)3-6(9)14-4;/h1,6-7,10H,2-3H2,(H,12,13);/q;+1/p-1/b4-1-;/t6-,7-;/m1./s1
Provided is a process for purification o...
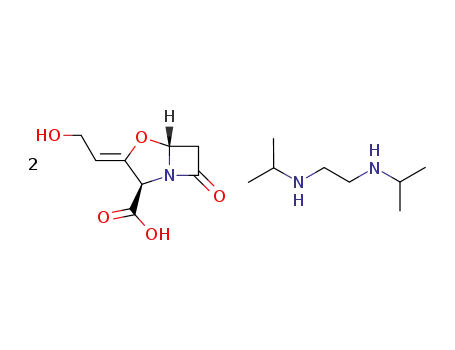
N,N'-diisopropylethylenediammonium diclavulanate

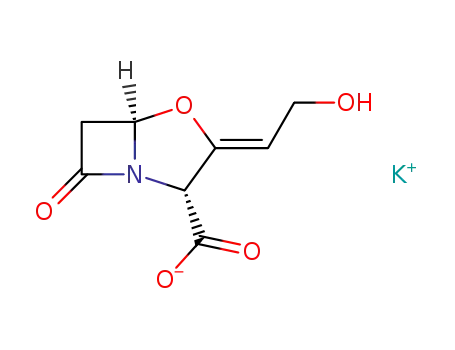
potassium clavulanate
| Conditions | Yield |
|---|---|
|
With
potassium 2-ethylhexanoate;
In
water; isopropyl alcohol;
at 0 - 10 ℃;
for 1h;
|
76% |
|
With
potassium 2-ethylhexanoate;
In
water; isopropyl alcohol;
|
70% |
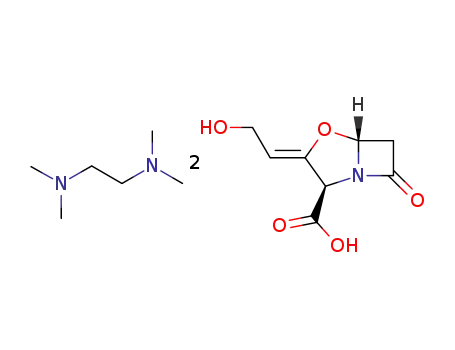
N,N,N',N'-tetramethylethylenediammonium diclavulanate


potassium clavulanate
| Conditions | Yield |
|---|---|
|
With
potassium 2-ethylhexanoate;
In
water; isopropyl alcohol;
at 20 ℃;
for 0.5h;
|
75% |
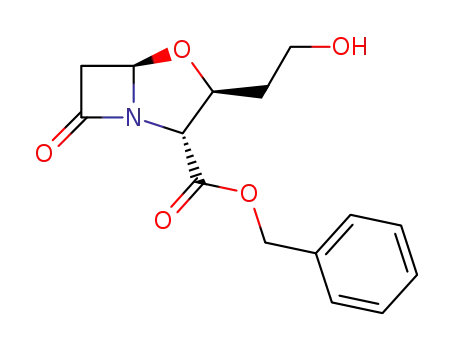
(2R,3S,5R)-benzyl-1-aza-3-(2'-hydroxyethyl)-4-oxa-7-oxobicyclo<3,2,0>heptane-2-carboxylate
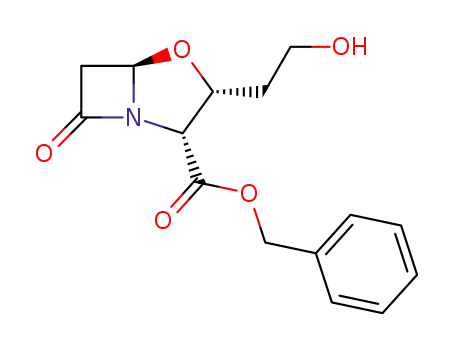
(2R,3R,5R)-benzyl-1-aza-3-(2'-hydroxyethyl)-4-oxa-7-oxobicyclo<3,2,0>heptane-2-carboxylate
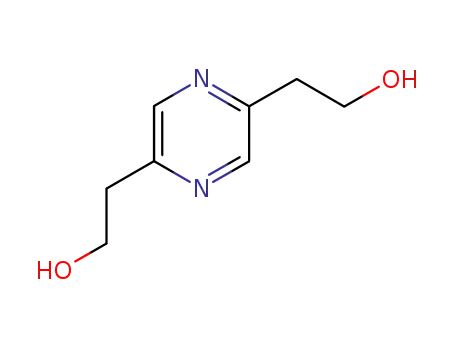
2,5-bis(2-hydroxyethyl)pyrazine
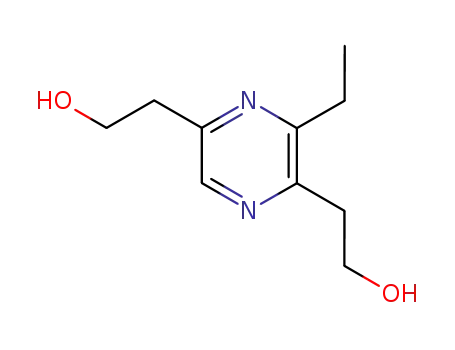
3-ethyl-2,5-di-(2-hydroxyethyl)-pyrazine
CAS:124750-99-8
CAS:3081-61-6
CAS:96-49-1
CAS:620-02-0