-
- 15383190639
- admin@86-ss.com
Your Location:Home >Products >Organic chemicals >620-02-0
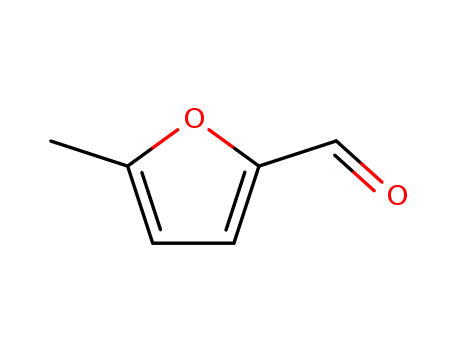

Product Details
|
Preparation |
From sucrose with HCl, followed by treating with stannous chloride; 5-Methyl furfural is produced by distillation of various methylpentoses with acid together. |
|
Synthesis Reference(s) |
Tetrahedron, 41, p. 3803, 1985 DOI: 10.1016/S0040-4020(01)91401-2Tetrahedron Letters, 24, p. 5441, 1983 DOI: 10.1016/S0040-4039(00)94107-8 |
|
Metabolic pathway |
The biotransformation of 5-methyl-2-furaldehyde is the conversion to 5-methylfuroylgycine and 5-methyl-2- furylmethylketone by rats. |
|
Physical properties |
colorless to light yellow liquid. soluble in benzene, toluene, carbon tetrachloride and other solvents, insoluble in water. |
|
Definition |
ChEBI: 5-methyl-2-furaldehyde is a member of furans and an aldehyde. It has a role as a Maillard reaction product, a human metabolite, an EC 2.2.1.6 (acetolactate synthase) inhibitor and a flavouring agent. |
|
Aroma threshold values |
Detection: 6 ppm |
|
Taste threshold values |
Taste characteristics at 50 ppm: sweet, brown, caramellic, grain, maple-like. |
|
General Description |
5-Methylfurfural is formed during the photoexposition of ranitidine hydrochloride. It is employed as potential age marker for Madeira wine. It is a volatile compound present in Lavandula stoechas, Lavandula angustifolia and Lavandula angustifolia x latifolia unifloral honeys. |
InChI:InChI=1/C6H6O2/c1-5-2-3-6(4-7)8-5/h2-4H,1H3
The renewable basic chemical 5-hydroxyme...
One-pot synthesis of furans from various...
A green approach for the conversion of 5...
2,5-Dimethylfuran (DMF) is one of the mo...
The dehydration of several sugars, inclu...
The role of amino acids and α-dicarbonyl...
The invention relates to the technical f...
The Vilsmeier reagent (VR), first report...
Herein, a robust catalytic system was de...
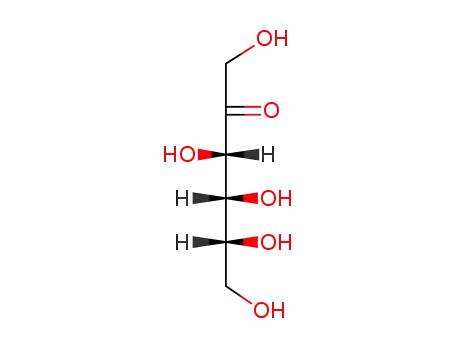
D-Fructose

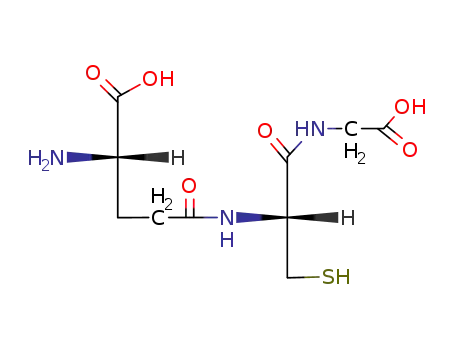
GLUTATHIONE

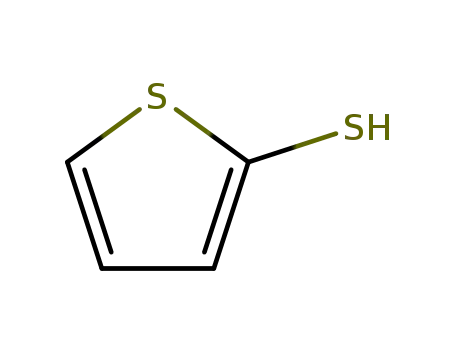
Thiophene-2-thiol

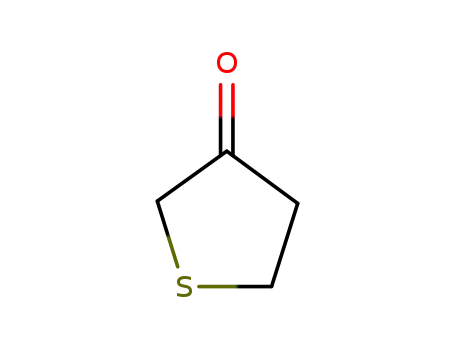
Tetrahydrothiophen-3-one

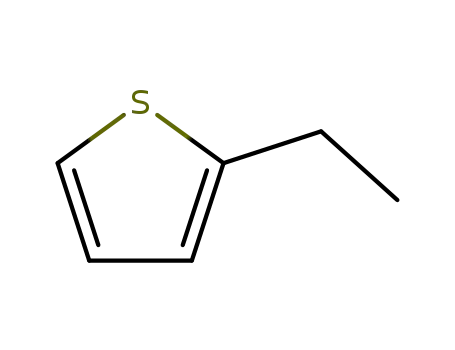
2-ethylthiophene

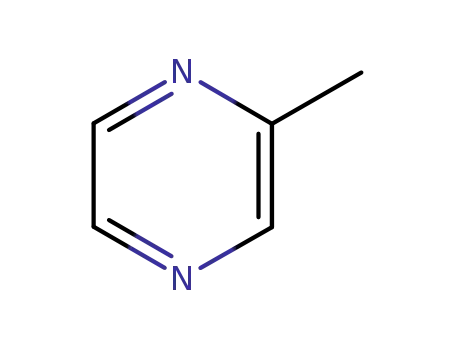
2-Methylpyrazine

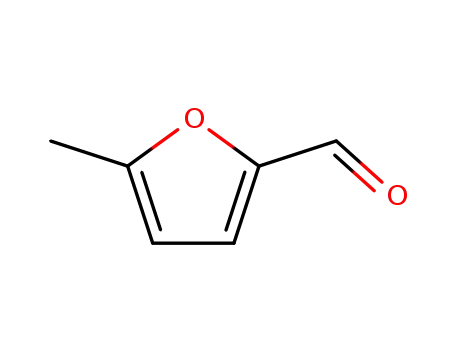
5-Methylfurfural

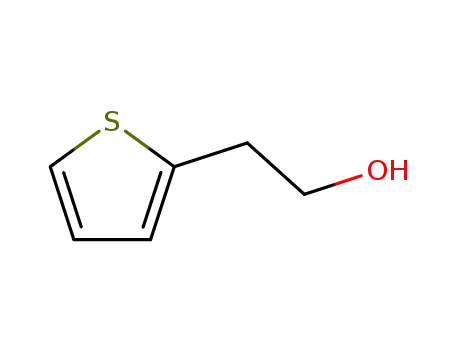
2-thiophenethanol

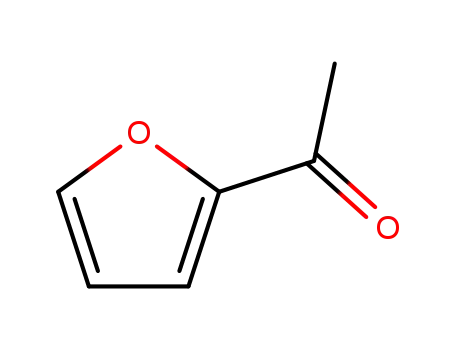
1-(2-furyl)-1-ethanone

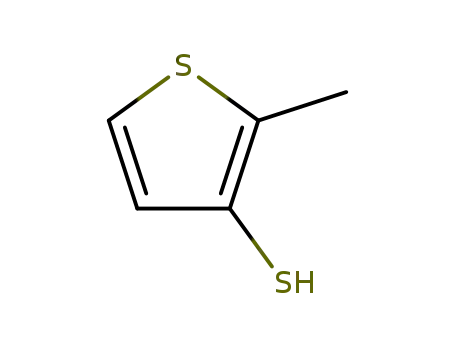
2-methylthiophene-3-thiol

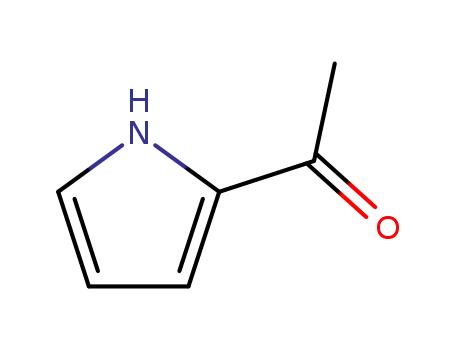
2-Acetylpyrrole

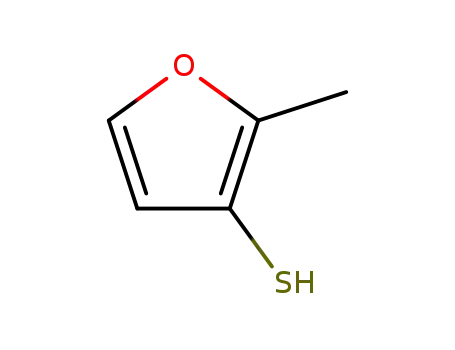
2-methylfuran-3-thiol
| Conditions | Yield |
|---|---|
|
In
water;
at 160 ℃;
for 2h;
pH=7.5;
|
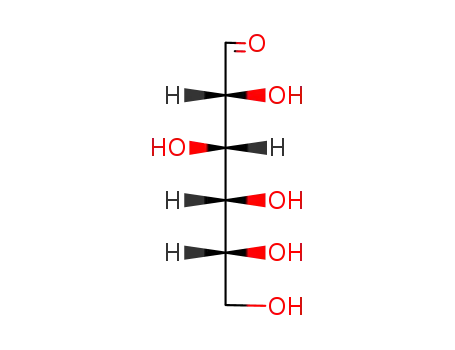
D-glucose


GLUTATHIONE

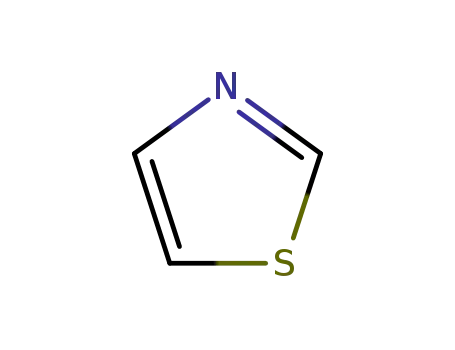
1,3-thiazole


Thiophene-2-thiol


Tetrahydrothiophen-3-one

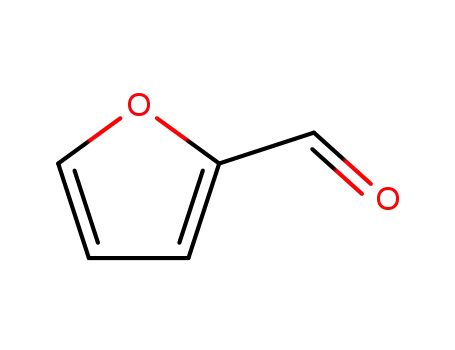
furfural

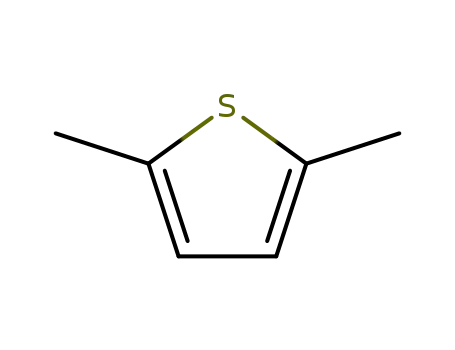
2,5-DIMETHYLTHIOPHENE


5-Methylfurfural


1-(2-furyl)-1-ethanone


2-methylthiophene-3-thiol


2-Acetylpyrrole


2-methylfuran-3-thiol
| Conditions | Yield |
|---|---|
|
In
water;
at 160 ℃;
for 2h;
pH=7.5;
|
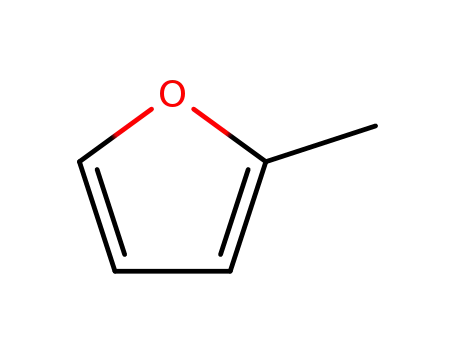
2-methylfuran

hydrogen cyanide
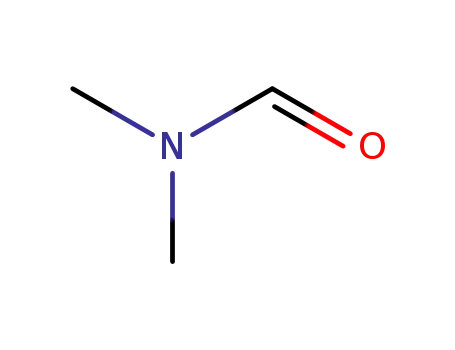
N,N-dimethyl-formamide
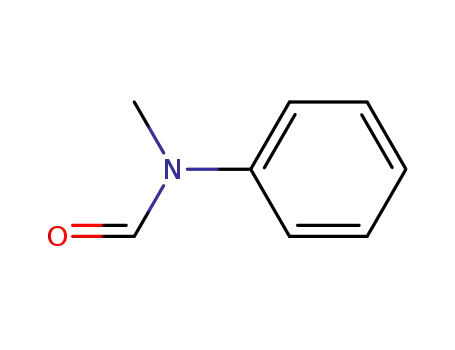
N-methyl-N-phenylformamide
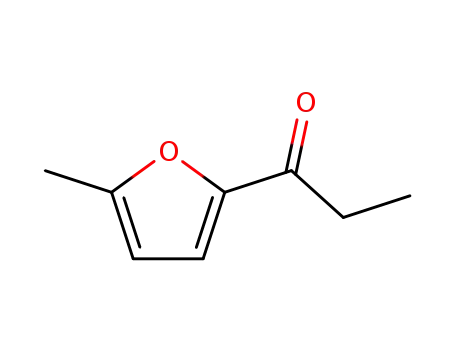
5-methyl-2-propionylfuran
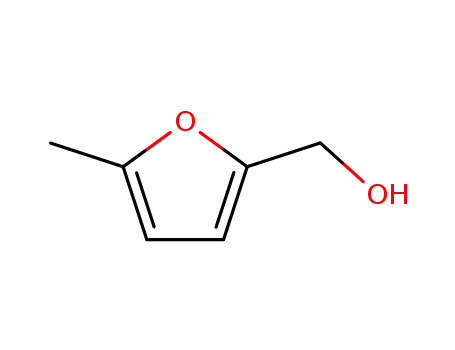
2-hydroxymethyl-5-methylfuran
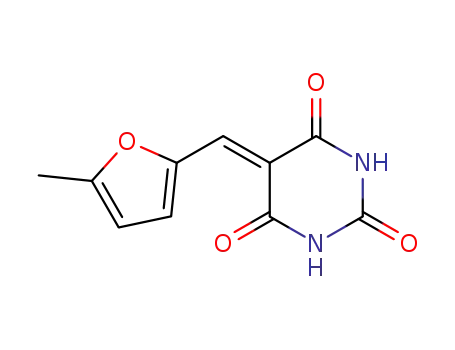
5-(5-methyl-furan-2-ylmethylene)-pyrimidine-2,4,6-trione
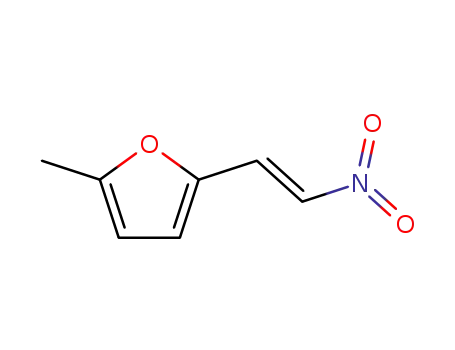
2-(5-methyl-2-furyl)-1-nitroethylene
CAS:3081-61-6
CAS:85070-48-0
CAS:61177-45-5
CAS:100-42-5