-
- 15383190639
- admin@86-ss.com
Your Location:Home >Products >Organic chemicals >123-08-0

Product Details
|
Chemical Description |
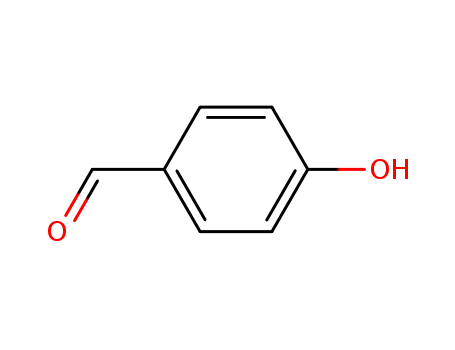
4-hydroxybenzaldehyde is an organic compound with a hydroxyl group and an aldehyde group. |
|
Chemical Composition and Structure |
4-Hydroxybenzaldehyde (4-HBA) is an organic compound belonging to the class of hydroxybenzaldehydes. It is characterized by a benzene ring with a hydroxyl group (-OH) and an aldehyde group (-CHO) attached at the para position. |
|
Sources |
4-Hydroxybenzaldehyde is found naturally in plants such as Gastrodia elata (Tianma) and Vanilla planifolia (vanilla orchid). It can also be synthesized chemically. |
|
Mechanism of Action |
4-Hydroxybenzaldehyde has been studied for its various biological activities, including its ability to inhibit GABA transaminase, modulate bacterial metabolism, and act as a flavoring agent. Its mechanism of action involves interactions with specific molecular targets, such as enzymes and receptors. |
InChI:InChI=1/C7H6O2/c8-5-6-1-3-7(9)4-2-6/h1-5,9H
-
Liquid-phase oxidation of p-cresol over ...
Mechanistic proposals and predictions ma...
Radical copolymerization of N-vinylpyrro...
Thermolytic cleavage of t-butyl esters a...
The para selectivity of the Reimer-Tiema...
Dimethylsulfoxide (DMSO) oxidizes benzyl...
Burkholderia gladioli is a Gram-negative...
Partial photocatalytic oxidation of sali...
The first synthesis of polyflavanostilbe...
A high-yield synthesis of p-hydroxybenza...
Cancer cells generally possess higher le...
This is a first report of highly efficie...
In contrast to N-protected tyrosine deri...
A composite of graphene oxide (GO) with ...
A selsctive attack of dichlorocarbene at...
-
The enzymatic formation of p-hydroxybenz...
The disposal of millions of tons of coal...
Two new merohexaprenoids, halicloic acid...
-
Supported iron catalysts are active for ...
The synthetic compound 4′,7-dihydroxyfla...
Horseradish Peroxidase (HRP) is a commer...
-
(2S)-Tyrosine (5) is incorporated stereo...
Through topological rationalization, a z...
Copper and manganese were found to be tw...
The reaction products of the photocataly...
-
A model compound, 4-(4-hydroxypheny])met...
4-(4-Hydroxyphenyl)-5-(4-hydroxyphenylme...
-
This study establishes the applicability...
In 0.1 M phosphate buffer, pH 3.0, and a...
The electrochemical oxidation of p-creso...
Two C-glucosides of resveratrol dimers (...
Photoactive yellow protein (PYP) is a ba...
Covalent organic frameworks (COFs) have ...
Devising artificial photoenzymes for abi...
Methods to activate the relatively stabl...
The efficient SBA-15 supported silver ca...
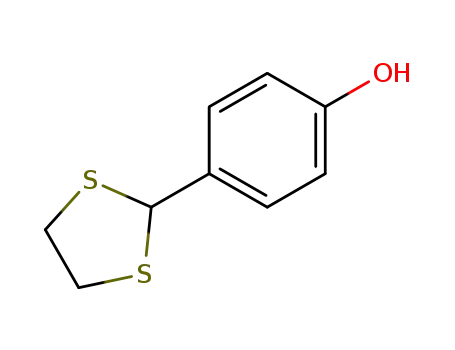
4-(1,3-dithiolan-2-yl)phenol

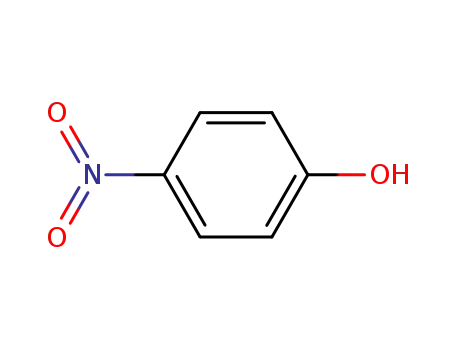
4-nitro-phenol

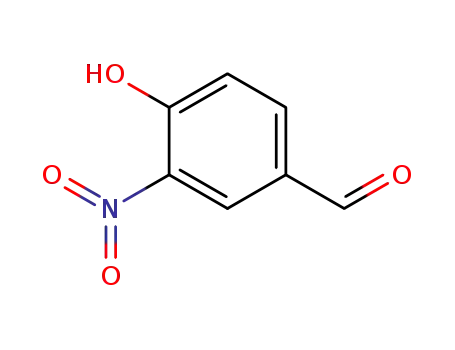
4-hydroxy-3-nitrobenzaldehyde

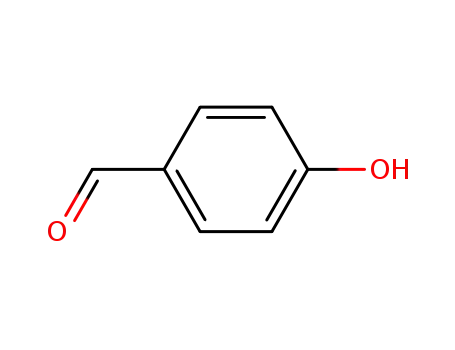
4-hydroxy-benzaldehyde

4-[1,3]dithiolan-2-yl-2-nitro-phenol
| Conditions | Yield |
|---|---|
|
With
bismuth(III) nitrate; water;
In
benzene;
at 20 ℃;
for 8h;
Product distribution;
|
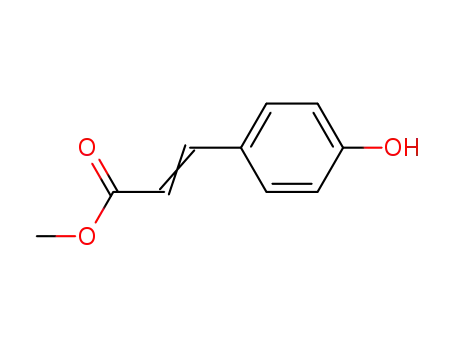
methyl p-hydroxycinnamate


4-nitro-phenol

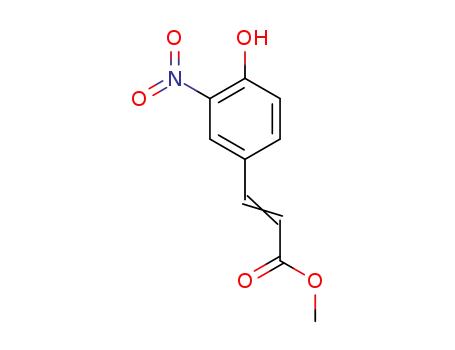
3‐nitro‐p‐coumaric acid methyl ester

C10H11ClO4


4-hydroxy-benzaldehyde
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; water; dihydrogen peroxide; sodium hydroxide; sodium nitrite;
In
aq. buffer;
|
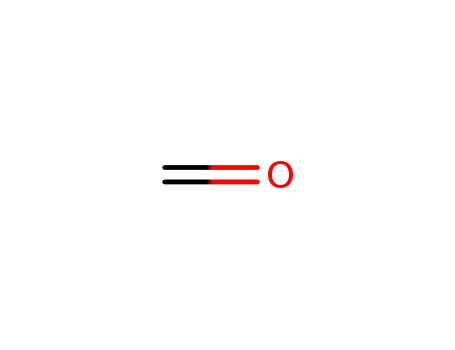
formaldehyd
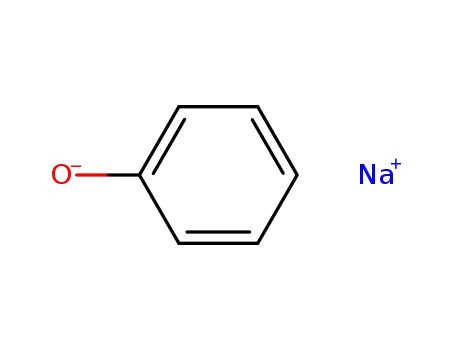
sodium phenoxide
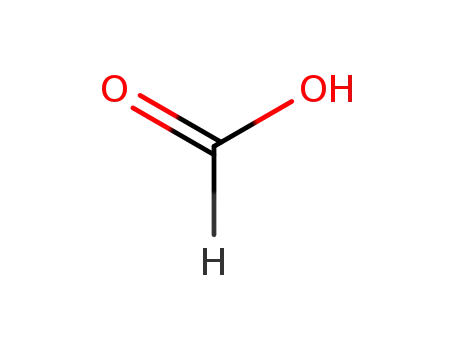
formic acid
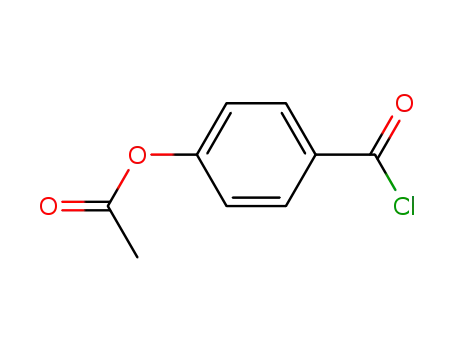
4-acetoxybenzoyl chloride
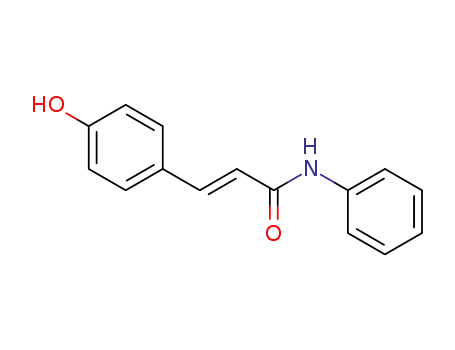
(E)-3-(4-hydroxyphenyl)-N-phenylacrylamide
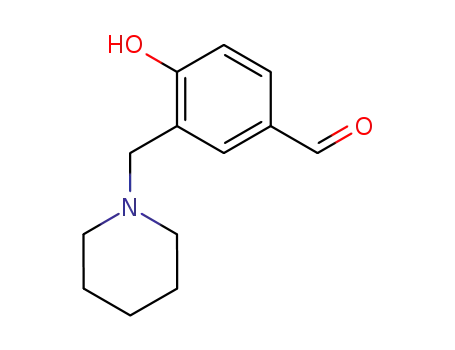
4-hydroxy-3-(piperidin-1-ylmethyl)benzaldehyde
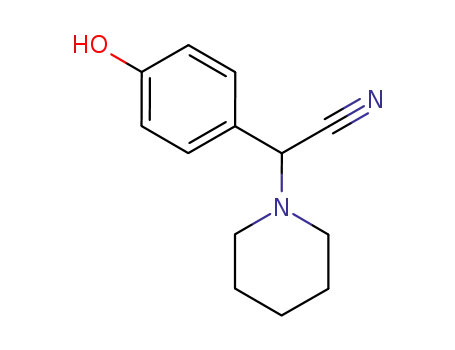
2-(4-hydroxyphenyl)-2-(piperidin-1-yl)acetonitrile
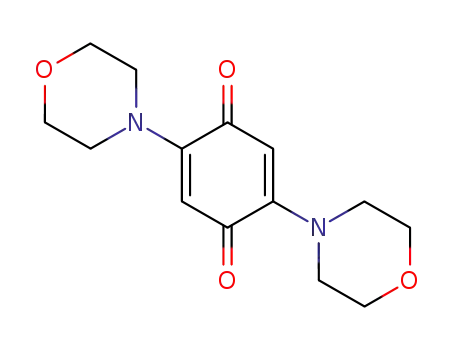
2,5-dimorpholinocyclohexa-2,5-diene-1,4-dione
CAS:3081-61-6
CAS:85070-48-0
CAS:7647-17-8
CAS:7664-38-2