-
- 15383190639
- admin@86-ss.com
Your Location:Home >Products >Pharmaceutical intermediate >7647-17-8

Product Details
|
Uses and Mechanism of Action |
In cancer therapy: |
|
Production Methods |
CsCl is typically synthesized by reacting cesium metal or cesium carbonate with hydrochloric acid. It can also be obtained as a byproduct of certain industrial processes. |
|
Analysis Method |
The properties of CsCl and CsCl-containing materials can be analyzed using various techniques, including XRD, XPS, SEM, UV-visible spectroscopy, and conductivity measurements, to understand their structure, stability, and performance. |
|
General Description |
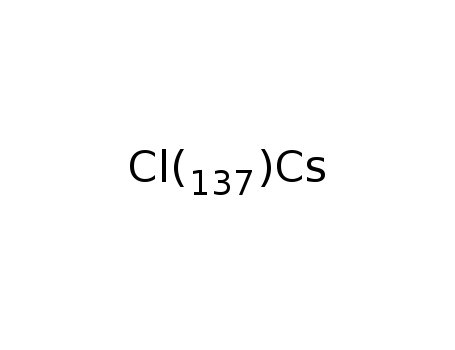
Cesium chloride is a chemical compound with the formula CsCl. It is a salt that is composed of cesium and chlorine ions in a 1:1 ratio. Cesium chloride is widely used in laboratory research for the preparation of density gradients in centrifugation, as well as for the purification of DNA. It is also used in the preparation of cesium-based compounds and as a source of cesium ions in a variety of chemical reactions. In addition, cesium chloride has been investigated for its potential in cancer therapy, as it has been shown to disrupt the ionic balance in cancer cells, leading to their destruction. However, |
InChI:InChI=1/ClH.Cs/h1H;/q;+1/p-1
All-inorganic halide perovskites are pro...
Ion exchange of layered alkali titanates...
The facile axial ligand exchange propert...
CsCl-NdCl3 is the next of binary MCl-NdC...
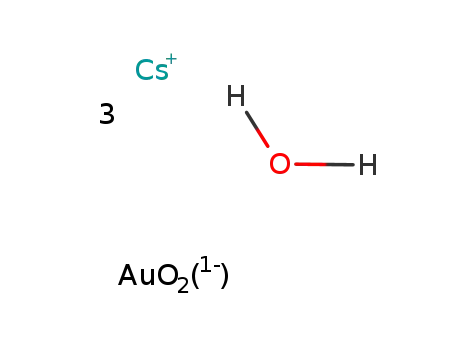
cesium aurate(III)


cesium chloride

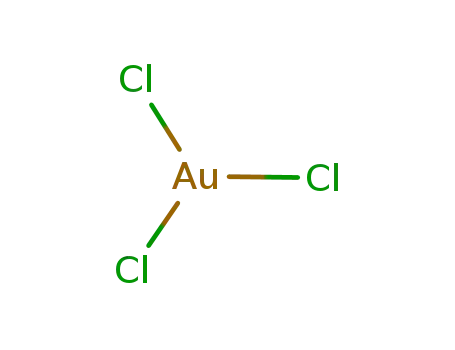
gold(III) chloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
|
|
|
With
HCl;
|
Cs(1+)*NbCl6(1-)=Cs[NbCl6]


cesium chloride

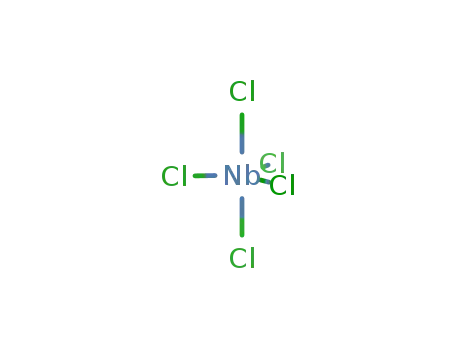
niobium pentachloride
| Conditions | Yield |
|---|---|
|
In
neat (no solvent);
heating to 508°C in N2-stream;;
|
n-Butyl chloride
caesium
caesium methoxide

rubidium chloride

caesium bromide
cesium hexafluoroarsenate
caesium tetrafluoroborate
CAS:85070-48-0
CAS:195875-84-4
CAS:1317-33-5
CAS:123-08-0