-
- 15383190639
- admin@86-ss.com
Your Location:Home >Products >Organic chemicals >77-93-0
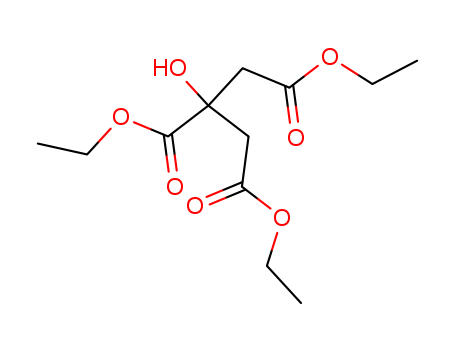

Product Details
|
Production Methods |
Triethyl citrate is prepared by the esterification of citric acid and ethanol in the presence of a catalyst. |
|
Preparation |
By esterification of ethyl alcohol with citric acid. |
|
General Description |
Triethyl citrate is a safe direct food additive. |
|
Pharmaceutical Applications |
Triethyl citrate and the related esters acetyltriethyl citrate, tributyl citrate, and acetyltributyl citrate are used to plasticize polymers in formulated pharmaceutical coatings. The coating applications include capsules, tablets, beads, and granules for taste masking, immediate release, sustained-release, and enteric formulations. Triethyl citrate is also used as a direct food additive for flavoring, for solvency, and as a surface active agent. |
|
Safety Profile |
Moderately toxic by intraperitoneal route. Mildly toxic by ingestion and inhalation. Combustible liquid when exposed to heat or flame. To fight fire, use dry chemical, CO2. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS and CITRIC ACID. |
|
Safety |
Triethyl citrate is used in oral pharmaceutical formulations and as a direct food additive. It is generally regarded as a nontoxic and nonirritant material. However, ingestion of large quantities may be harmful. LD50 (mouse, IP): 1.75 g/kg LD50 (rat, IP): 4 g/kg LD50 (rat, oral): 5.9 g/kg LD50 (rat, SC): 6.6 g/kg |
|
storage |
Triethyl citrate should be stored in a closed container in a cool, dry location. When stored in accordance with these conditions, triethyl citrate is a stable product. |
|
Incompatibilities |
Triethyl citrate is incompatible with strong alkalis and oxidizing materials. |
|
Regulatory Status |
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (oral capsules and tablets). Included in the Canadian List of Acceptable Nonmedicinal Ingredients. |
InChI:InChI=1/C12H20O7/c1-4-17-9(13)7-12(16,11(15)19-6-3)8-10(14)18-5-2/h16H,4-8H2,1-3H3
The invention discloses a citrate plasti...
The invention relates to a method for pr...
A process for the preparation of methyls...
The invention designs a synthetic method...
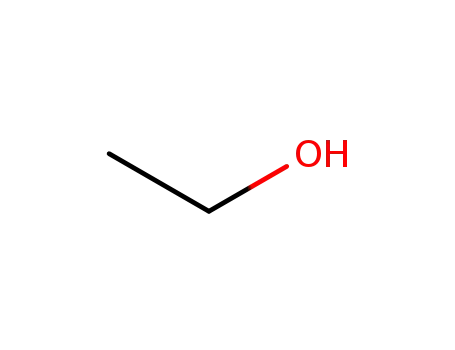
ethanol

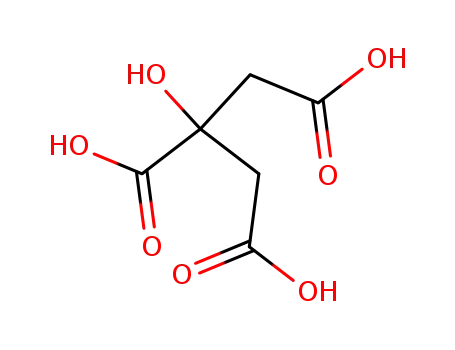
citric acid

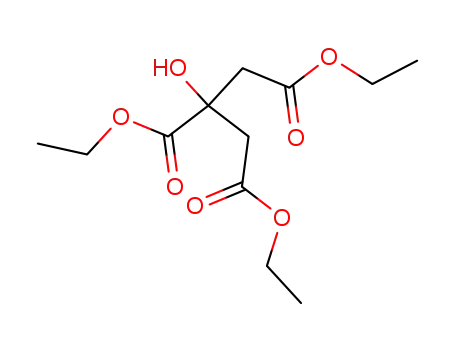
citric acid triethyl ester
| Conditions | Yield |
|---|---|
|
ethanol; With dicyclohexyl-carbodiimide; at 20 ℃; for 36h; Large scale;
citric acid; at 35 ℃; for 6h; Temperature; Autoclave; Large scale;
|
96% |
|
With sulfonated graphene; In neat (no solvent); at 90 ℃; for 4h;
|
94% |
|
With boric acid; benzenesulfonic acid; at 85 - 90 ℃; for 3h;
|
93.26% |
|
With sodium hydrogensulfate monohydrate; at 80 - 90 ℃; Reagent/catalyst; Temperature;
|
90.2% |
|
With toluene-4-sulfonic acid; In toluene; at 90 - 110 ℃; for 4h;
|
86.3% |
|
With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; at 75 ℃; for 3h;
|
85% |
|
With carbonic acid dimethyl ester; at 180 ℃; for 5h; under 10343.2 Torr; Reagent/catalyst; Temperature; Inert atmosphere;
|
55.8% |
|
With hydrogenchloride;
|
|
|
With hydrogenchloride; in mehreren Stufen;
|
|
|
With sulfuric acid; 1,2-dichloro-ethane;
|
|
|
With folate;
|
|
|
With sulfuric acid; toluene;
|
|
|
With sulfuric acid; at 0 - 80 ℃; for 4h;
|
|
|
With toluene-4-sulfonic acid; at 60 - 135 ℃; Concentration; Temperature; Further stages; Autoclave;
|
|
|
With nanometer intercalation hydrotalcite catalyst with [PW11CoO39]5- polyacid; at 100 - 105 ℃; Reagent/catalyst; Temperature;
|
|
|
With benzene; azeotrope Destillation;
|
|
|
With toluene-4-sulfonic acid; In cyclohexane; water; at 110 ℃; for 4h;
|

ethanol


citric acid

Diethyl 2-methylsuccinate


citric acid triethyl ester
| Conditions | Yield |
|---|---|
|
With hydrogen; In water; at 200 ℃; for 6h; under 3000.3 Torr;
|
19 %Spectr. |
diethyl 1,3-acetonedicarboxylate
formic acid ethyl ester

ethanol

citric acid
rac-4-oxocyclopentane-1,2-dicarboxylic acid
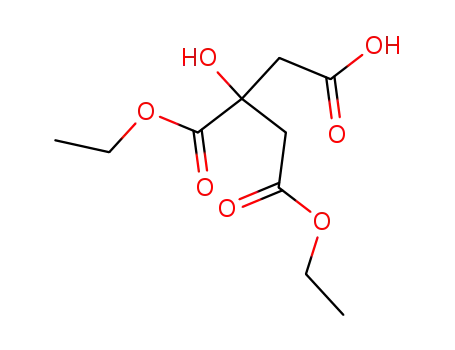
3-Hydroxy-3,4-bis(ethoxycarbonyl)butanoic acid
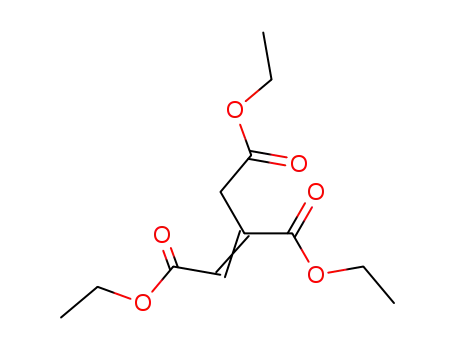
Diethyl-3-(ethoxycarbonyl)pent-2-endioat
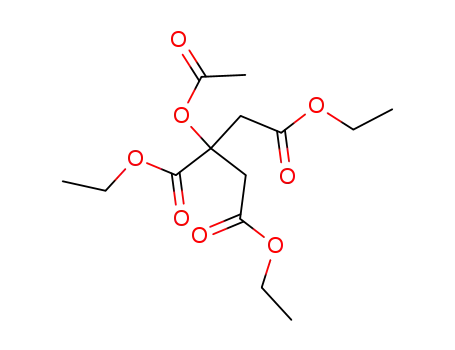
triethyl O-acetylcitrate
CAS:3081-61-6
CAS:85070-48-0
CAS:527-07-1
CAS:123-94-4