-
- 15383190639
- admin@86-ss.com
Your Location:Home >Products >Pharmaceutical intermediate >34157-83-0
Product Details
|
Pharmacological effects |
Tripterine is a natural product with a variety of biological activity. It has a strong antioxidant effect, anti-cancer neovascularization effect as well as anti-rheumatoid effect. It is mainly isolated from the root bark of the celastraceae plant, Tripterygium wilfordii. It is one of the active ingredients contained in the rheumatoid-treatment drug Tripterygium tablet, Tripterygium Glycosides tablets and other preparations. The major activity and pharmacological effects of Tripterine: Cytotoxic activity. In vitro, it has non-specific cytotoxic activity against P388 and a group of human cancer cell lines. Immune regulation. It could significantly inhibit the formation of hemolytic plaque cells in mouse spleen cells and significantly inhibit the mice delayed-type hypersensitivity. Anti-inflammatory effect. At a dose of 0.5mg/kg, it could result in significant inhibition of the formation of rat cotton granuloma. At a dose of 0.1~1.0μg/mL, it could suppress the production of PGE2 induced by the yeast sugar; at a dose of 1.0μg/mL, it can inhibit the macrophage phagocytosis. The anti-oxidation effect of tripterine is 15 times that of tocopherol with an IC50 of 7μM. Inhibit the peroxidation occurring inside and outside of the mitochondrial membrane, direct removing the free radicals. Tripterine can extend the sleep time of mice triggered by the pentobarbital sodium. Immunosuppressive effect: inhibit the spleen cell proliferation of mice induced by PHA, ConA and LPS, further inhibiting the lymphocyte proliferation. It can inhibit the in vitro sperm fertilization capacity of guinea pig with the activity being significantly stronger than acetic acid gossypol. Anti-arthritic effect, it will inhibit the activity of interleukin-1 of inside and outside the mouse intraperitoneal macrophages, inhibit the production of interleukin-2 by mouse spleen cells and lower the level of PGE2 produced by rabbit synovial cells. Source: lookchem Editorial. |
|
Biological Activity |
Antioxidant and anti-inflammatory agent. Potently inhibits lipid peroxidation in mitochondria and inhibits TNF- α -induced NF κ B activation. Also shown to inhibit topoisomerase II activity in vitro (IC 50 = 7.41 μ M). |
|
Biochem/physiol Actions |
Celastrol is a potent antioxidant, and anti-inflammatory agent. It is a novel HSP90 inhibitor (disrupts Hsp90/Cdc37 complex), that exhibits anticancer (anti-angiogenic - suppresses VEGFR expression); antioxidant (inhibits lipid peroxidation) and anti-inflammatory activity (suppresses iNOS and inflammatory cytokine production). |
|
Application |
Celastrol is an effective proteasome inhibitor. It has been confirmed that it is capable of inducing apoptosis of cancer cells through inhibiting proteasome activity. |
|
Definition |
ChEBI: A pentacyclic triterpenoid that is 24,25,26-trinoroleana-1(10),3,5,7-tetraen-29-oic acid bearing an oxo substituent at position 2, a hydroxy substituent at position 3 and two methyl groups at positions 9 and 13. An antioxidant and anti-inflammatory agent. otently inhibits lipid peroxidation in mitochondria and inhibits TNF-alpha-induced NFkappaB activation. Also shown to inhibit topoisomerase II activity in vitro (IC50 = 7.41 muM). |
|
General Description |
This substance is a primary reference substance with assigned absolute purity (considering chromatographic purity, water, residual solvents, inorganic impurities). The exact value can be found on the certificate. Produced by PhytoLab GmbH & Co. KG |
InChI:InChI=1/C29H38O4/c1-17-18-7-8-21-27(4,19(18)15-20(30)23(17)31)12-14-29(6)22-16-26(3,24(32)33)10-9-25(22,2)11-13-28(21,29)5/h7-8,15,22,31H,9-14,16H2,1-6H3,(H,32,33)/t22-,25-,26-,27+,28-,29+/m1/s1
Celastrol is one of the most studied nat...
C31H44O5S

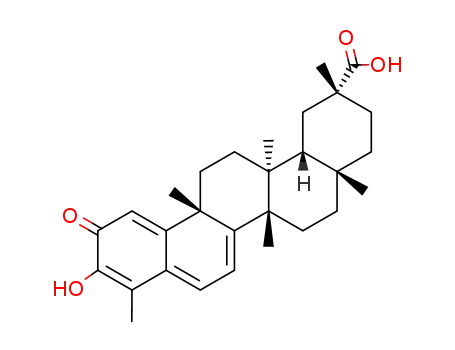
celastrol

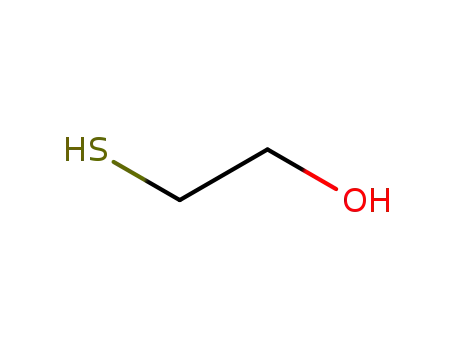
2-hydroxyethanethiol
| Conditions | Yield |
|---|---|
|
In aq. phosphate buffer; dimethyl sulfoxide; Equilibrium constant;
|
C39H55N3O10S

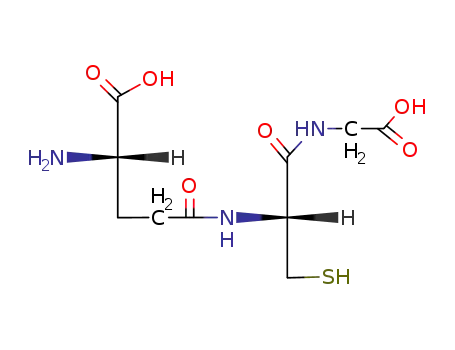
GLUTATHIONE


celastrol
| Conditions | Yield |
|---|---|
|
In aq. phosphate buffer; dimethyl sulfoxide; Equilibrium constant;
|
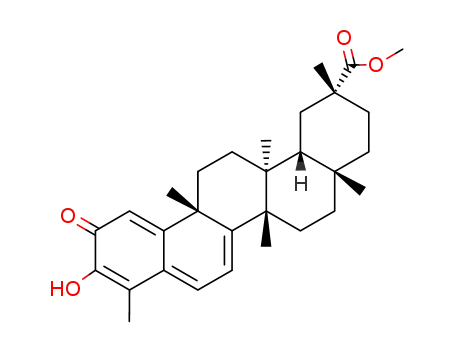
pristimerin
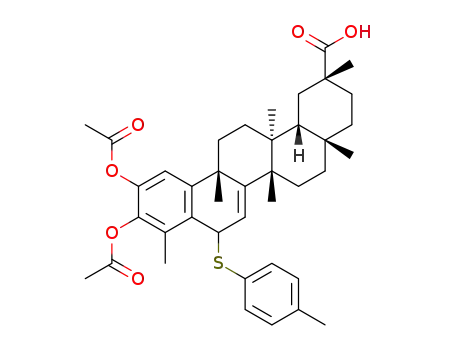
(2R,4aS,6aS,12bS,14aS,14bR)-10,11-diacetoxy-2,4a,6a,9,12b,14a-hexamethyl-8-(p-tolylthio)-1,2,3,4,4a,5,6,6a,8,12b,13,14,14a,14b-tetradecahydropicene-2-carboxylic acid
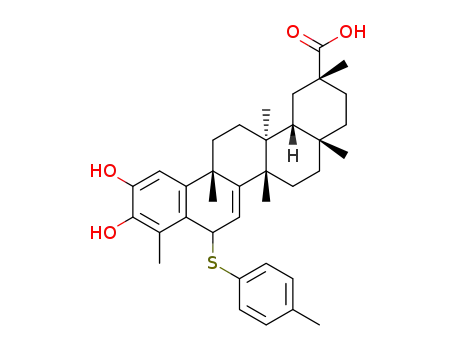
C36H46O4S
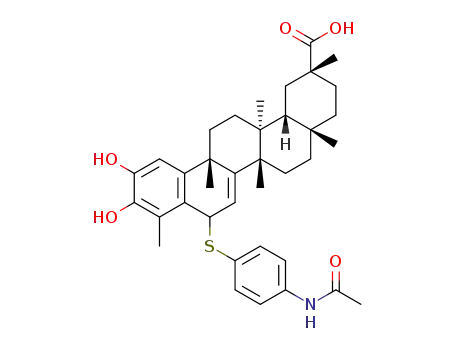
C37H47NO5S
CAS:3081-61-6
CAS:85070-48-0
CAS:14644-61-2
CAS:484-12-8