-
- 15383190639
- admin@86-ss.com
Your Location:Home >Products >Organic chemicals >97-88-1
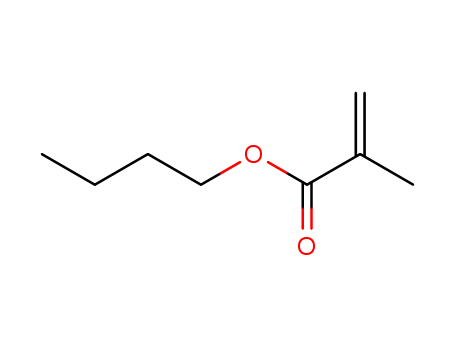

Product Details
|
Production Methods |
n-Butyl methacrylate is produced by the reaction of methacrylic acid or methyl methacrylate with butanol . |
|
Air & Water Reactions |
Flammable. Sensitive to moisture. Insoluble in water. |
|
Reactivity Profile |
Butyl methacrylate reacts exothermically with acids. Reacts with oxidizing agents. Strong oxidizing acids may cause a reaction that is sufficiently exothermic to ignite the reaction products. Heat is generated with caustic solutions. Generates flammable hydrogen with alkali metals and hydrides. Polymerizes easily . |
|
Health Hazard |
Inhalation may cause nausea because of offensive odor. Contact with liquid causes irritation of eyes and mild irritation of skin. Ingestion causes irritation of mouth and stomach. |
|
Fire Hazard |
Behavior in Fire: Containers may explode due to polymerization. |
|
Chemical Reactivity |
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: May occur upon exposure to heat; Inhibitor of Polymerization: 9-15 ppm monomethyl ether of hydroquinone; 90-120 ppm hydroquinone. |
|
Safety Profile |
Moderately toxic by intraperitoneal route. Mddly toxic by ingestion, inhalation, and skin contact. An experimental teratogen. Experimental reproductive effects. A skin irritant. Flammable liquid when exposed to heat or flame. Explosive in the form of vapor when exposed to heat or flame. Violent polymerization can be caused by heat, moisture, oxidizers. To fight fire, use foam, dry chemical, CO2. When heated to decomposition it emits acrid smoke and irritating fumes. |
|
Potential Exposure |
Forms an explosive mixture with air. Unless inhibitor is maintained at the proper level, oxidizers, heat, ultraviolet light, contamination, or moisture may cause polymerization. May accumulate static electrical charges and cause ignition of its vapors. |
|
Shipping |
UN2227 Butyl methacrylate, stabilized, Hazard Class: 3; Labels: 3—Flammable liquid. |
|
Purification Methods |
Purify as for butyl acrylate. [Beilstein 2 IV 1525.] |
|
Incompatibilities |
Forms an explosive mixture with air. Unless inhibitor is maintained at the proper level, oxidizers, heat, ultraviolet light, contamination, or moisture may cause polymerization. May accumulate static electrical charges and cause ignition of its vapors |
|
General Description |
A clear colorless liquid. Flash point 130°F. Less dense (7.5 lb / gal) than water and insoluble in water. Hence floats on water. Vapors heavier than air. Used to make resins adhesives, and oil additives. |
InChI:InChI=1/C8H14O2/c1-4-6(2)5-7(3)8(9)10/h6H,3-5H2,1-2H3,(H,9,10)/p-1
Manganese, the third most abundant trans...
A synergistic catalytic method combining...
The present invention relates to a metho...
The present invention relates to a prepa...
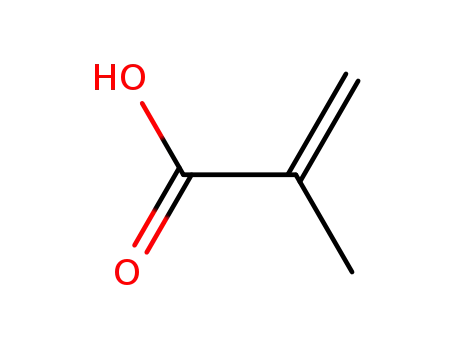
poly(methacrylic acid)

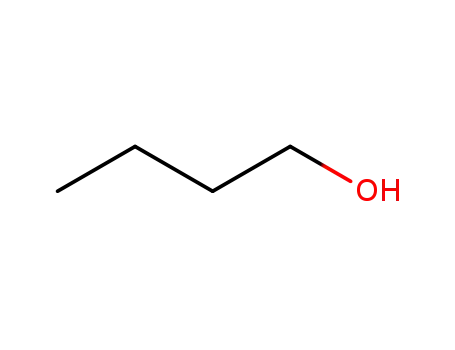
butan-1-ol

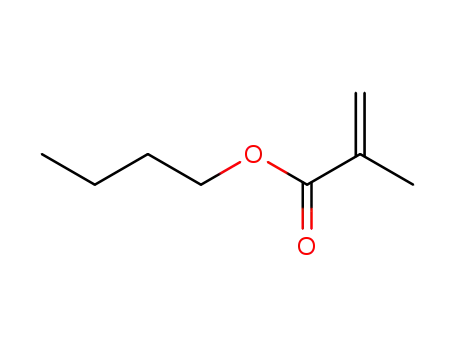
2-methyl-butyl acrylate
| Conditions | Yield |
|---|---|
|
sulfopolyphenylene ketone;
at 140 ℃;
under 3800 Torr;
Kinetics;
Product distribution;
continuous process in stainless tube reactor; studied at various temperature and at different feed rates of the raw material;
|
97.3% |
|
With
titanium catalyst;
at 115 ℃;
for 1h;
|
92.11% |
|
With
sulfuric acid; hydroquinone; benzene;
|
|
|
With
toluene-4-sulfonic acid;
In
benzene;
Heating;
|
|
|
With
dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;
In
dichloromethane;
at 20 ℃;
|
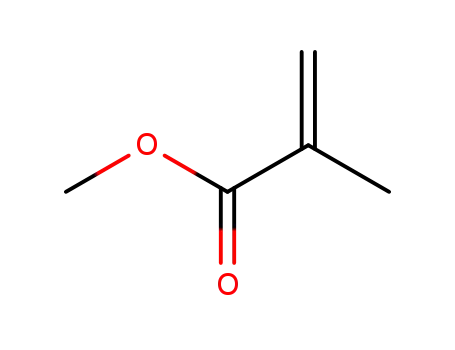
methacrylic acid methyl ester


butan-1-ol


2-methyl-butyl acrylate
| Conditions | Yield |
|---|---|
|
With
air;
titanium(III) triisopropoxide; acetylacetone functionalised polymethylstyrene; chlorot;
for 4h;
Heating;
|
85% |
|
With
titanium catalyst;
at 115 ℃;
for 1h;
Temperature;
|
71.03% |
|
tetrabutoxytitanium;
at 115 ℃;
for 1h;
Product distribution / selectivity;
|
63.8% |
|
With
sodium butanolate;
|
|
|
tetrabutoxytitanium;
at 100 - 130 ℃;
Industry scale;
|
|
|
With
10H-phenothiazine; 4-methoxy-phenol;
at 115 ℃;
flow conditions;
|
|
|
Industrial scale;
|
|
|
With
tetrabutoxytitanium; TEMPOL;
at 111 ℃;
for 2h;
|
10.3 g |
|
With
tetrabutoxytitanium; TEMPOL;
at 115 ℃;
for 2h;
|
|
|
With
10H-phenothiazine;
In
water;
at 115 ℃;
Temperature;
|
|
|
at 64.5 - 91.9 ℃;
Temperature;
Concentration;
|
|
|
With
sulfuric acid;
at 115 ℃;
|
|
|
With
Mn(II) supported on silica;
at 80 ℃;
for 24h;
Reagent/catalyst;
Glovebox;
Inert atmosphere;
Sealed tube;
|
96 %Spectr. |
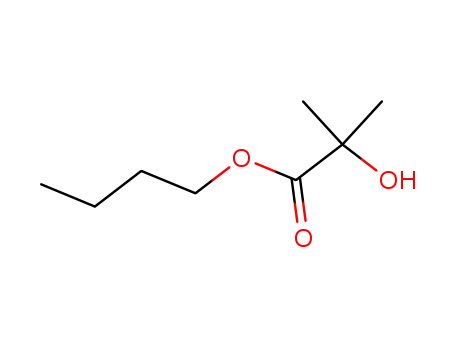
α-hydroxy-isobutyric acid butyl ester
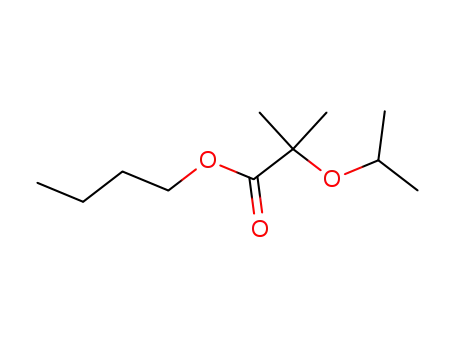
α-isopropoxy-isobutyric acid butyl ester
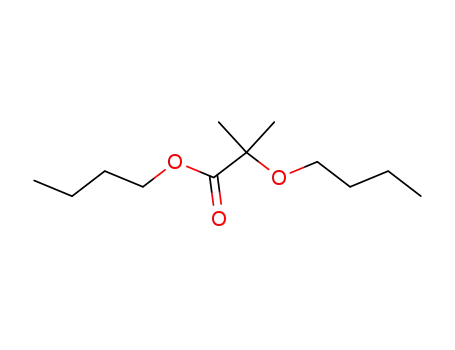
α-butoxy-isobutyric acid butyl ester
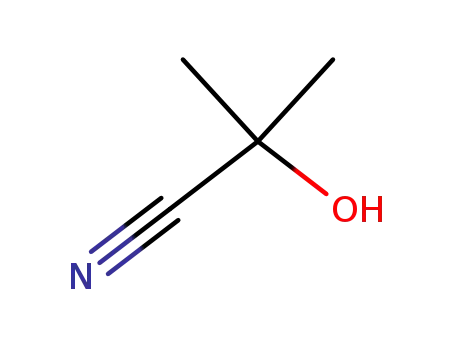
2-hydroxy-2-methylpropanenitrile

poly(methacrylic acid)
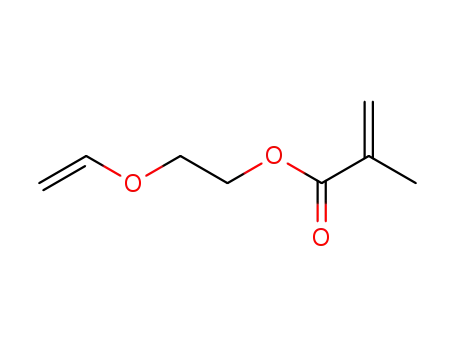
2-(vinyloxy)ethyl methacrylate
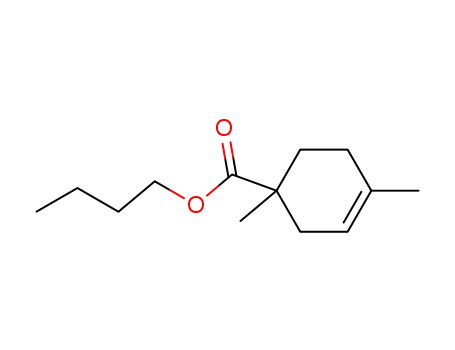
1,4-dimethyl-cyclohex-3-enecarboxylic acid butyl ester
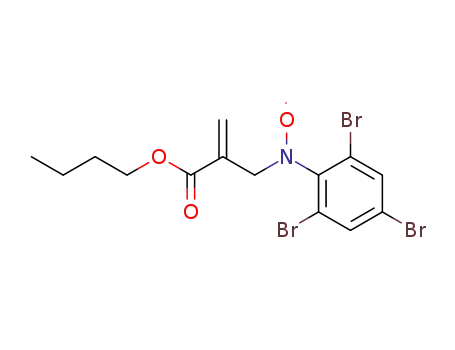
C14H15Br3NO3
CAS:3081-61-6
CAS:85070-48-0
CAS:95041-90-0
CAS:119515-38-7