-
- 15383190639
- admin@86-ss.com
Your Location:Home >Products >Organic chemicals >118-91-2

Product Details
|
General Description |
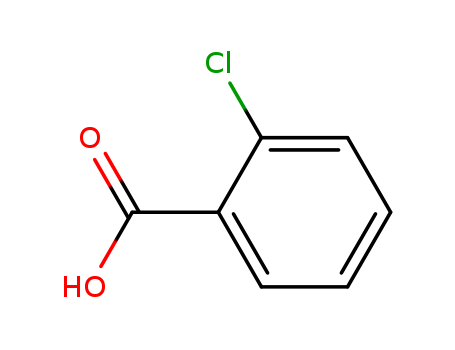
o-Chlorobenzoic acid is a simple organic compound with the chemical formula C7H5ClO2. It consists of a benzene ring bearing a carboxylic acid group and a chlorine atom at the ortho position. o-Chlorobenzoic acid is a white crystalline solid that is used in the synthesis of various pharmaceuticals, dyes, and agrochemicals. It is also used as an intermediate in the production of other chemicals. o-Chlorobenzoic acid is moderately toxic and may cause irritation to the skin, eyes, and respiratory system upon exposure. Additionally, it is not readily biodegradable and it poses a risk to the environment if released into water or soil. |
InChI:InChI=1/C7H5ClO2/c8-6-4-2-1-3-5(6)7(9)10/h1-4H,(H,9,10)/p-1
-
Photocatalytic mineralization of 2-chlor...
We report herein an easy oxidation proce...
Bismuth(III)mandelate catalyzes the oxid...
Chemiluminescence is observed in the the...
In the present work, the ability of two ...
Reaction of halogenobenzoic acids with b...
Cobalt carbonyl-catalyzed double-carbony...
Reductions and free radical cyclizations...
In the present work, a new method for th...
-
Zeolite imidazolate frameworks (ZIFs) ha...
The oxidation of aldehydes is an efficie...
As an efficient heterogenous N-heterocyc...
A one-pot procedure for deprotecting car...
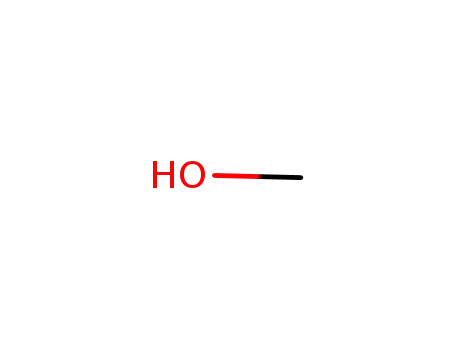
methanol

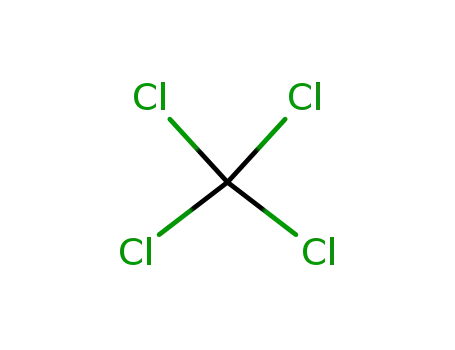
tetrachloromethane

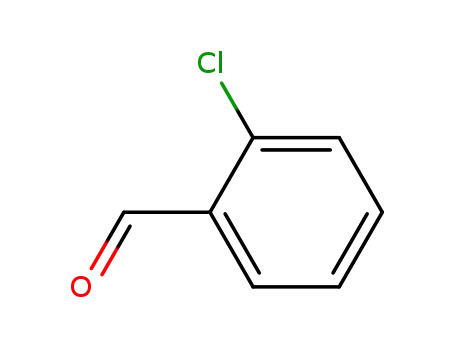
2-chloro-benzaldehyde

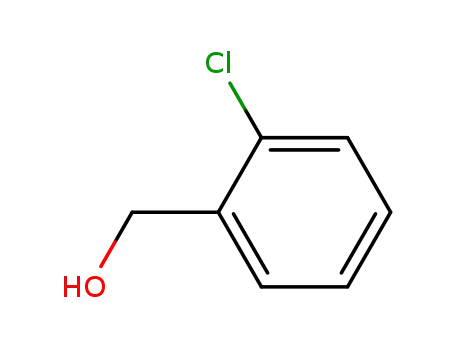
2-Chlorobenzyl alcohol

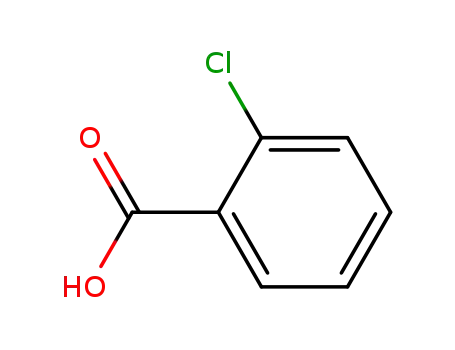
ortho-chlorobenzoic acid
| Conditions | Yield |
|---|---|
|
Kinetics; Disproportionierung;
|
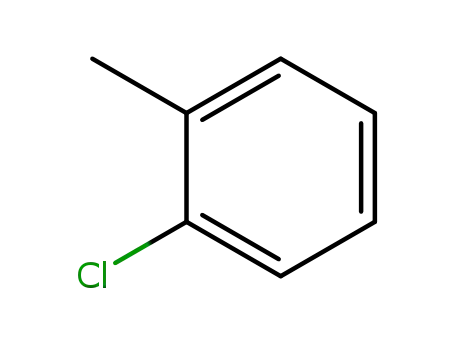
2-methylchlorobenzene


2-chloro-benzaldehyde

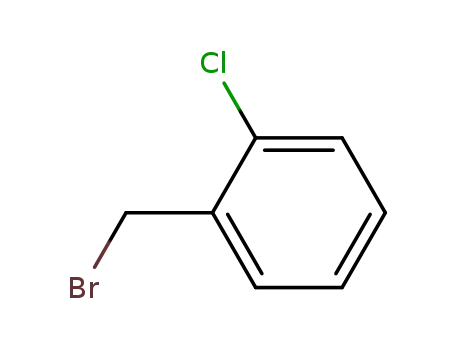
1-bromomethyl-2-chlorobenzene


ortho-chlorobenzoic acid
| Conditions | Yield |
|---|---|
|
With oxygen; cobalt(II) acetate; sodium bromide; In acetic acid; at 95 ℃; for 0.666667h; Kinetics; Mechanism; Rate constant; other time; other temperature; various concentrations of Co(OAc)2 and NaBr;
|
96% |
|
With oxygen; cobalt(II) acetate; sodium bromide; In acetic acid; at 95 ℃; for 0.666667h;
|
95% |
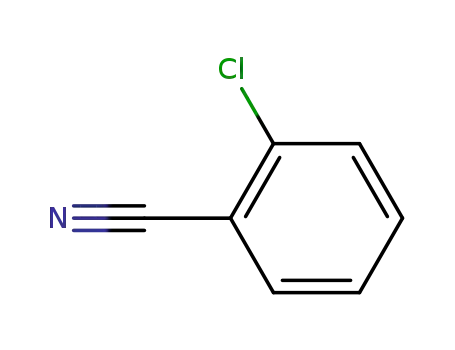
2-Chlorobenzonitrile
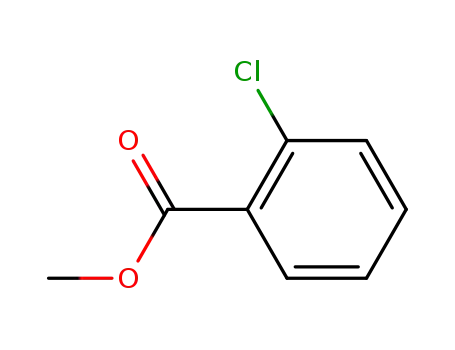
methyl chlorobenzoate
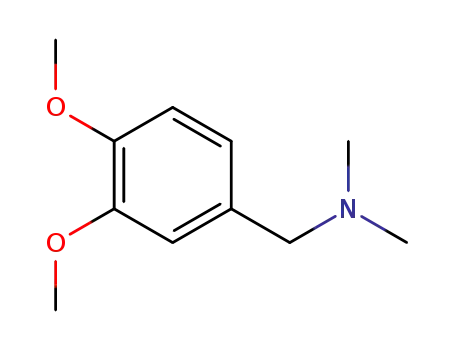
N,N-dimethyl-3,4-dimethoxybenzylamine
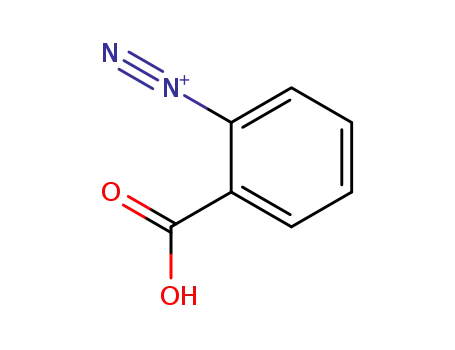
o-Carboxybenzenediazonium
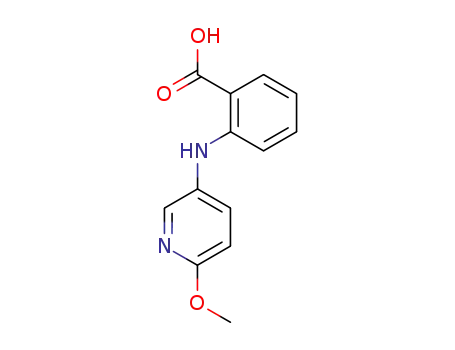
N-(6-methoxy-[3]pyridyl)-anthranilic acid
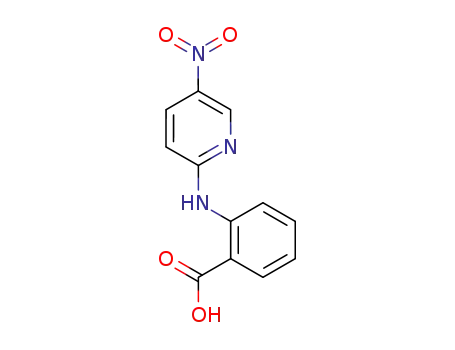
2-(5-nitro-pyridin-2-ylamino)-benzoic acid
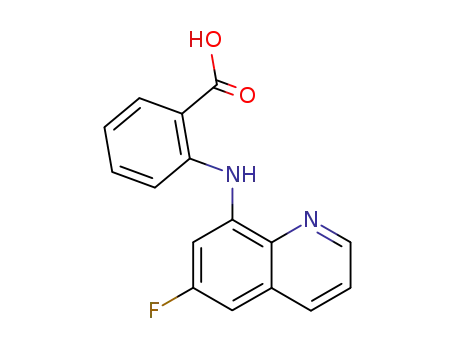
N-(6-fluoro-[8]quinolyl)-anthranilic acid
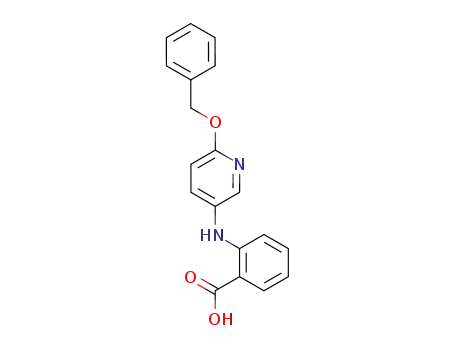
N-(6-benzyloxy-[3]pyridyl)-anthranilic acid
CAS:85070-48-0
CAS:195875-84-4
CAS:73-40-5
CAS:95041-90-0