-
- 15383190639
- admin@86-ss.com
Your Location:Home >Products >Organic chemicals >106-94-5
Product Details
|
General Description |
1-Bromopropane, also known as n-propyl bromide, is a colorless liquid with a sweet odor. It is commonly used as a solvent and a cleaning agent in various industrial processes, such as metal cleaning, electronics manufacturing, and pharmaceutical production. It is also used as an intermediate in the synthesis of pharmaceuticals and agrochemicals. However, 1-bromopropane is classified as a hazardous chemical due to its potential for causing damage to the nervous system, as well as concerns about its reproductive and developmental toxicity. Exposure to 1-bromopropane should be minimized and proper safety precautions should be implemented when handling this chemical in order to protect workers and the environment from its harmful effects. |
InChI:InChI=1/C3H7Br/c1-2-3-4/h2-3H2,1H3
-
The infrared multiple-photon decompositi...
-
-
-
The kinetics of n-propyl iodide with tet...
-
-
Microwave spectra of trans and gauche pr...
-
-
-
The kinetics of the reactions of n-C3H7,...
-
-
Metabolite profiling in anaerobic alkane...
This work reports a chemo-selective semi...
A series of new N-alkyl functionalised 6...
A method comprising: providing a first h...
(1-ethyl-propyl)-propyl ether

hydrogen bromide

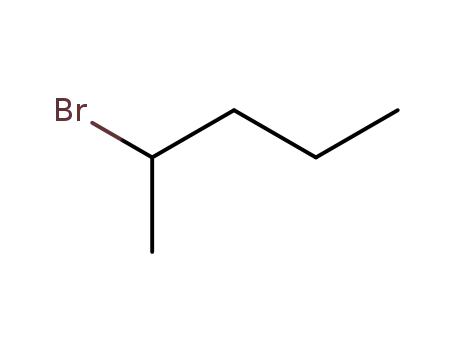
2-bromopentane

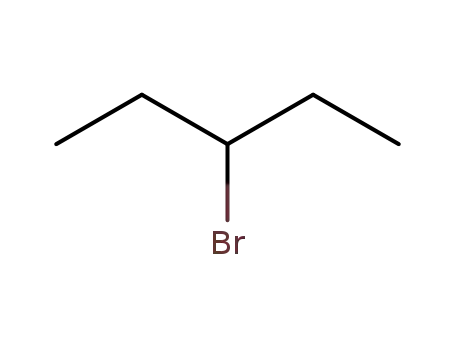
3-bromopentane

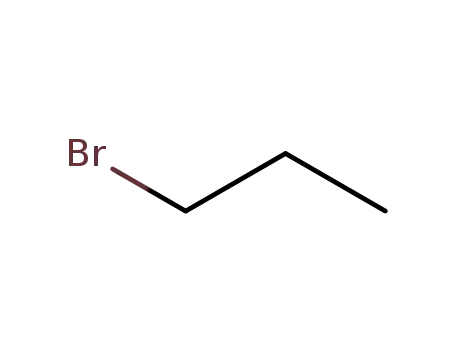
propyl bromide
| Conditions | Yield |
|---|---|
|
|
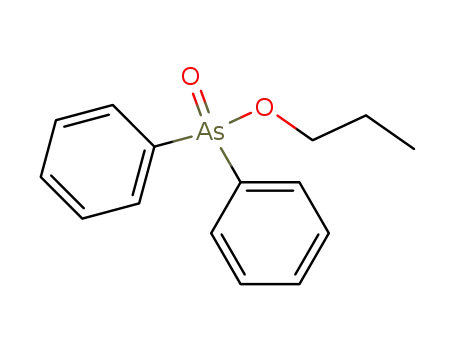
propyl diphenylarsinate

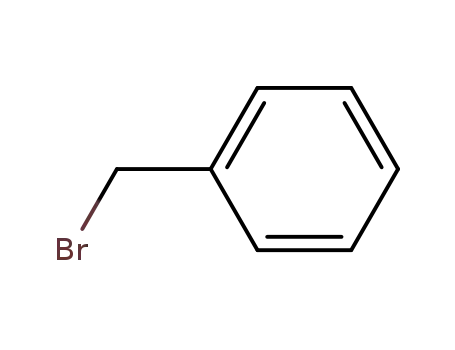
benzyl bromide

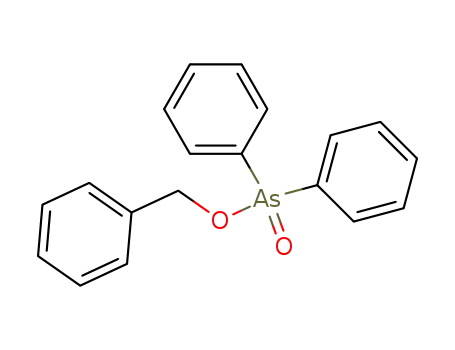
benzyl diphenylarsinate

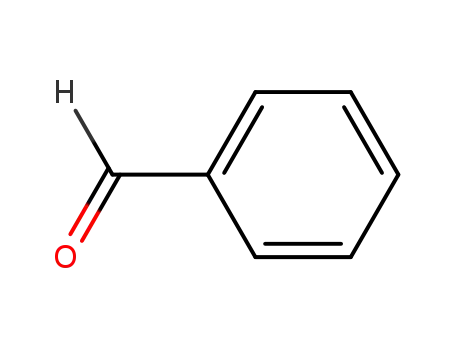
benzaldehyde

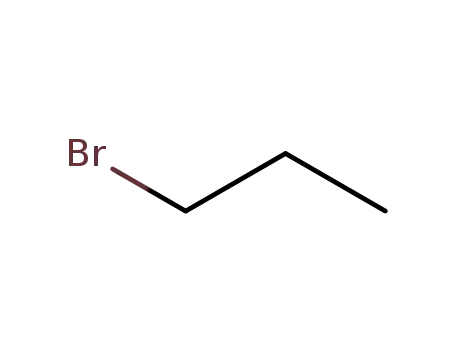
propyl bromide

C19H17AsO
| Conditions | Yield |
|---|---|
|
at 185 ℃;
for 1.5h;
Further byproducts given;
|
16.8% 74.5% |
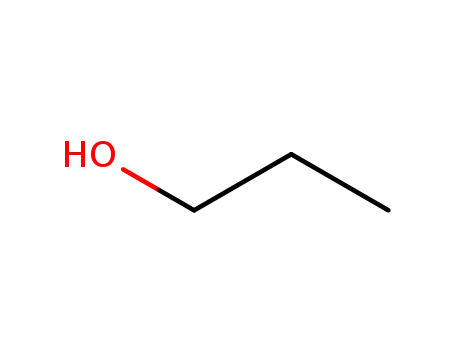
propan-1-ol

tri-n-propylamine
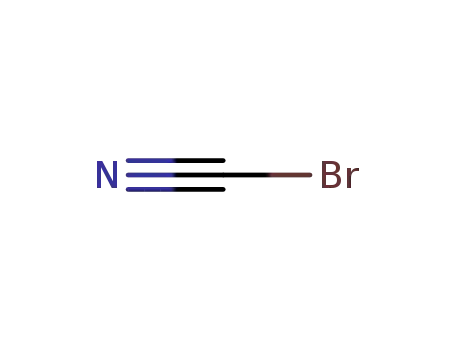
bromocyane
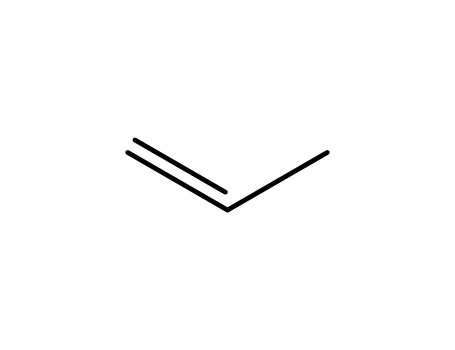
propene
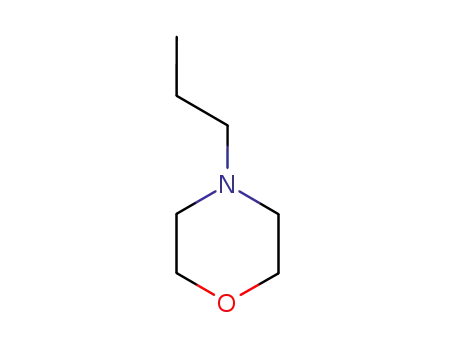
4-propyl-morpholine
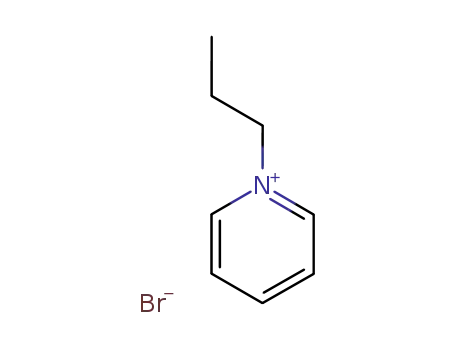
N-propylpyridinium bromide
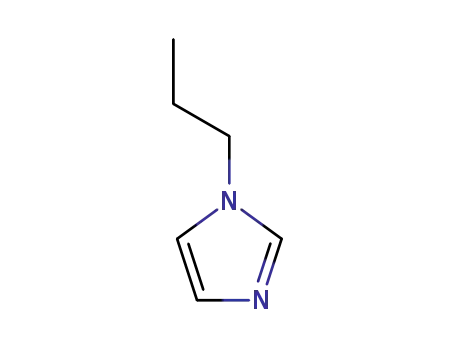
1-propyl-1H-imidazole
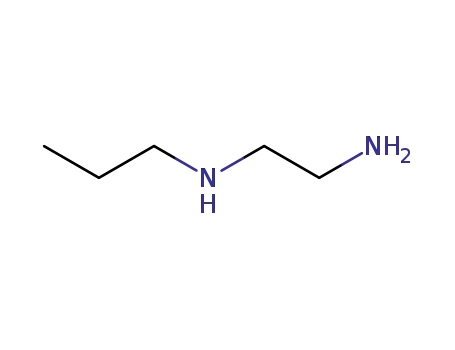
N-propylethane-1,2-diamine
CAS:3081-61-6
CAS:85070-48-0
CAS:71-30-7
CAS:56038-13-2