-
- 15383190639
- admin@86-ss.com
Your Location:Home >Products >Pharmaceutical intermediate >56-92-8

Product Details
|
Biological Activity |
Endogenous agonist at histamine receptors (H 1-4 ). Released from mast cells and basophils and exhibits inflammatory, vasodilatory and bronchoconstrictory activity. Stimulates gastric acid secretion and acts as a neurotransmitter in vivo . |
|
Biochem/physiol Actions |
Histamine dihydrochloride has been shown to activate nitric oxide synthetase and suppress or inhibit the generation of reactive oxygen species (ROS). Inhibition of ROS by histamine dihydrochloride allows activation of T cells and NK cells by IL-2. In a rat model, histamine dihydrochloride suppressed ROS generated by Kupffer cells through the H2 histamine receptor. |
|
Purification Methods |
The dihydrochloride crystallises from aqueous EtOH. The phosphate (2H3PO4) [51-74-1] has m 132-133o (from H2O). [Beilstein 25 III/IV 2049.] |
|
General Description |
Histamine Dihydrochloride is a derivative of histamine, which is implicated in tumor cell apoptosis and prevents the relapse of acute myeloid leukemia in patients. It prevents the formation of ROS (reactive oxygen species) and inhibits the activation of T cells and natural killer cells.Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. |
InChI:InChI=1/C5H9N3.ClH/c6-2-1-5-3-7-4-8-5;/h3-4H,1-2,6H2,(H,7,8);1H/p-1
The invention belongs to the technical f...
The invention discloses a synthesis meth...
of the invention The invention relates t...
The invention discloses a histamine dihy...
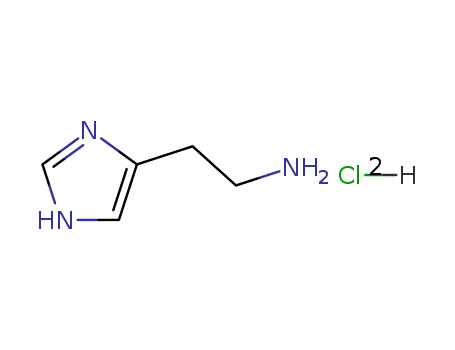
L-histidine

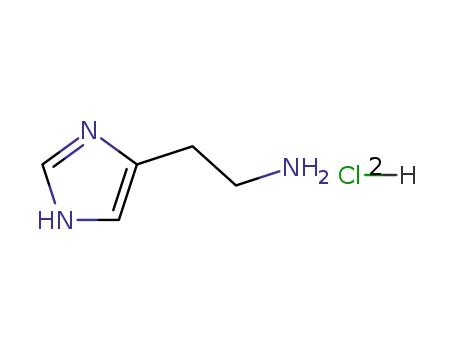
histamine dichloride
| Conditions | Yield |
|---|---|
|
L-histidine;
With
(R)-Carvone;
In
propan-1-ol;
at 190 ℃;
for 0.2h;
under 11251.1 Torr;
Sealed tube;
With
hydrogenchloride;
In
propan-1-ol; water;
at 190 ℃;
for 0.0833333h;
|
92% |
|
Multi-step reaction with 2 steps
1: propan-1-ol / 20 - 190 °C / Green chemistry
2: hydrogenchloride / propan-1-ol / 0.2 h / 190 °C / Green chemistry
With
hydrogenchloride;
In
propan-1-ol;
|
|
|
Multi-step reaction with 3 steps
1: copper(I) bromide / 10 h / 100 - 110 °C / Inert atmosphere; Darkness
2: hydrogenchloride / 30 °C
3: hydrogenchloride / methanol / 30 °C
With
hydrogenchloride; copper(I) bromide;
In
methanol;
|
Nα-Trityl-L-histidin


histamine dichloride
| Conditions | Yield |
|---|---|
|
Nα-Trityl-L-histidin;
With
(R)-Carvone;
In
propan-1-ol;
at 0 - 190 ℃;
Green chemistry;
With
hydrogenchloride;
In
propan-1-ol; water;
at 190 ℃;
for 0.0833333h;
Microwave irradiation;
Green chemistry;
|
86% |
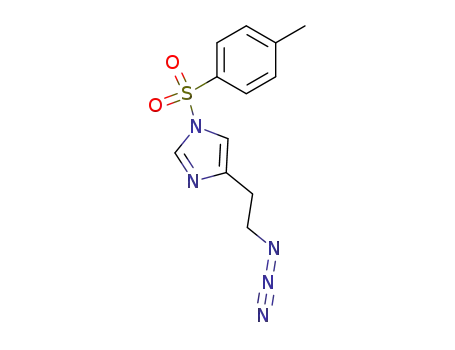
4-(2-Azido-ethyl)-1-(toluene-4-sulfonyl)-1H-imidazole
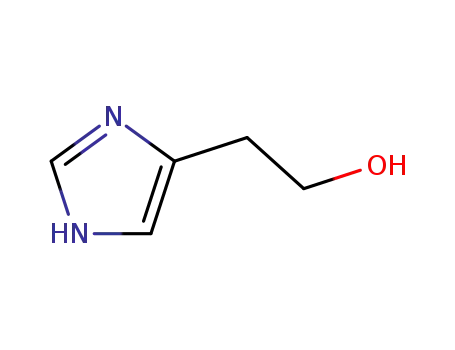
2-(1H-imidazol-4-yl)ethanol
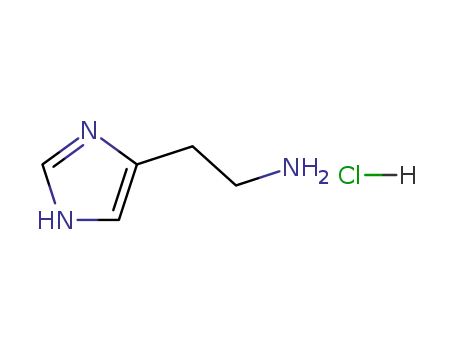
histamine hydrochloride
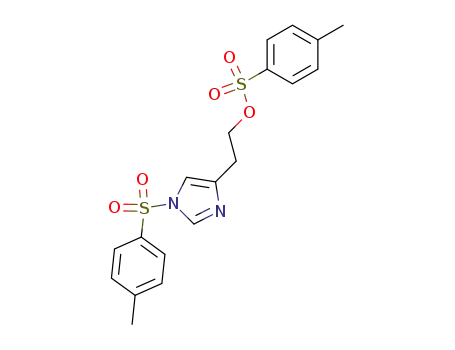
NO-bis-p-tolylsulphonylhistaminol
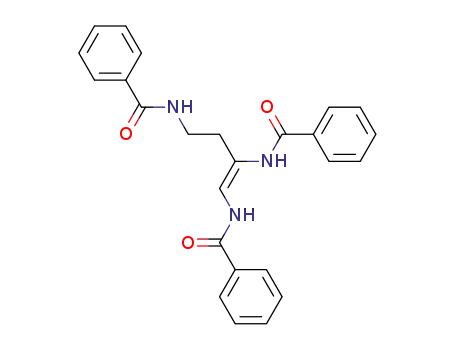
1,2,4-tris-(benzoilamino)-1-butene
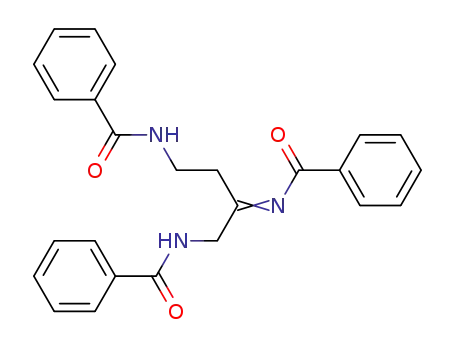
2-Benzoylimino-1,4-dibenzamido-butan
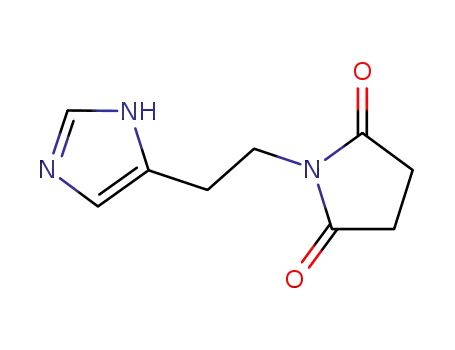
N-<2-(imidazol-4-yl)ethyl>succinimide
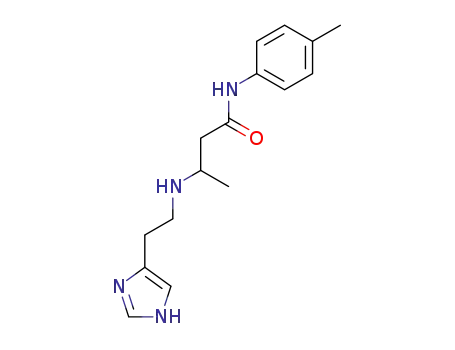
3-[2-(1H-Imidazol-4-yl)-ethylamino]-N-p-tolyl-butyramide
CAS:195875-84-4
CAS:417716-92-8
CAS:53-84-9
CAS:11138-66-2