-
- 15383190639
- admin@86-ss.com
Your Location:Home >Products >Organic chemicals >1310-73-2

Product Details
|
General Description |
Sodium hydroxide, also known as caustic soda, is a highly versatile and widely used inorganic compound. It is a strong and corrosive base that is odorless and highly soluble in water. It is commonly used in the manufacturing of soaps, detergents, and paper products, as well as in various industrial processes, such as in the production of aluminum, textiles, and petroleum products. Additionally, it is used in water treatment and as a cleaning agent. Sodium hydroxide is also a key ingredient in the production of biodiesel and as a pH regulator in various chemical processes. Despite its numerous applications, sodium hydroxide should be handled with caution, as it can cause severe burns and damage to skin and eyes upon contact. |
InChI:InChI=1/Na.H2O/h;1H2/q+1;/p-1/rHNaO/c1-2/h2H
The addition of NaH by ball milling is s...
Kinetics and equilibrium are studied on ...
A new thermochemical closed cycle of sod...
Single-crystalline TiOF2 was prepared by...
Aluminum hydroxide with gibbsite structu...
The reaction between Na and multilayer w...
The interaction of gaseous HOI with crys...
Na5[CuO2](OH)2 has been obtained as oran...
The crystal structure of the nonasodium ...
The heterogeneous reactions of HOBr with...
Rice husks (or rice hulls) pre-treated w...
An electrochemical technique has been de...
The uptake kinetics of HOBr on NaBr and ...
A key hydrolysis product of vanadocene d...
Honeycomb porous silicon (hp-Si) has bee...
To establish the structure of ferric ion...
Dark-blue sodium nitride, Na3N, was prep...
Methods and apparatus for generation of ...
A process for producing an alkali metal ...
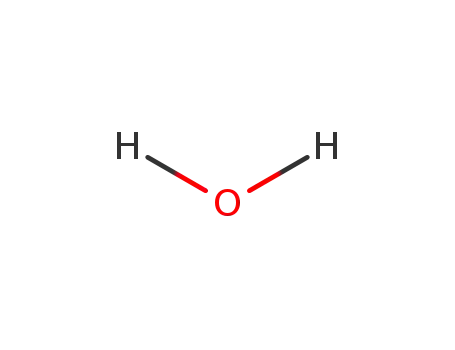
water

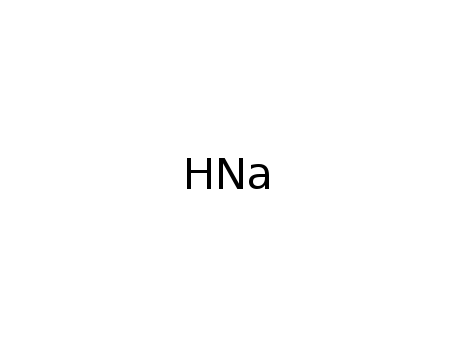
sodium hydride

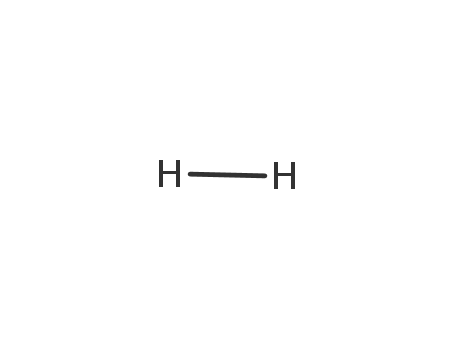
hydrogen


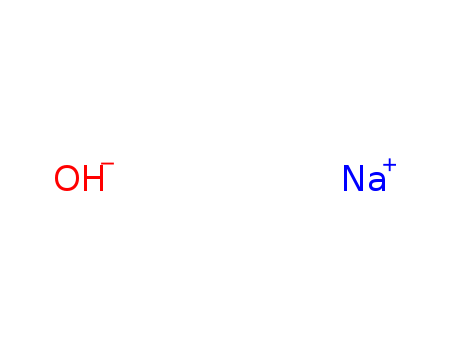
sodium hydroxide
| Conditions | Yield |
|---|---|
|
In neat (no solvent); exothermic react., react. enthalpy given;;
|
|
|
In neat (no solvent); exothermic react., react. enthalpy given;;
|
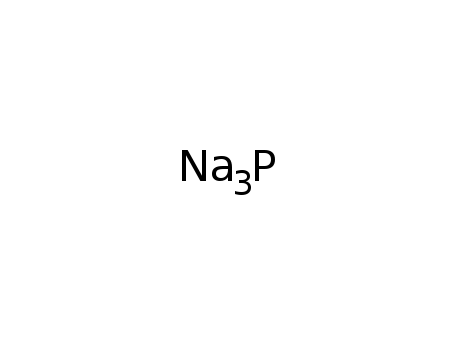
sodium phosphide


water

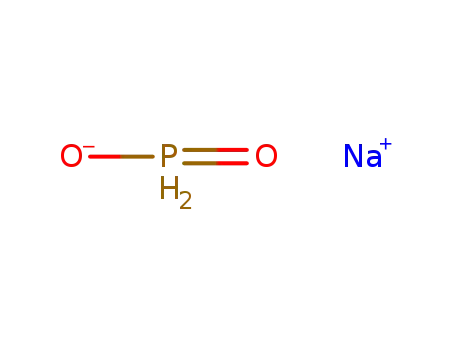
sodium hypophosphite

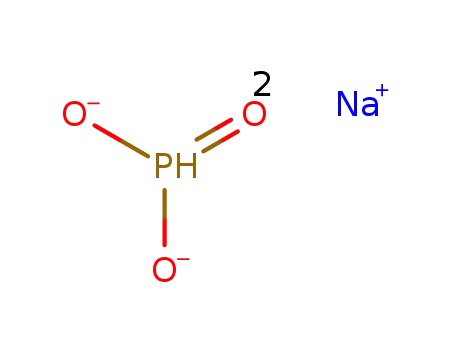
sodium phosphite


hydrogen

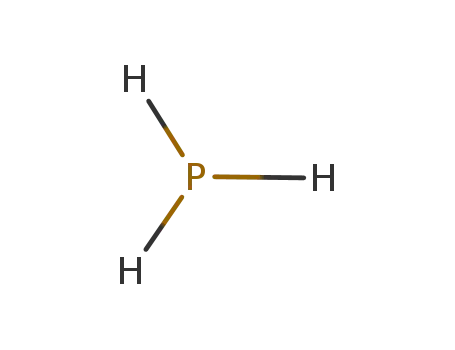
phosphan


sodium hydroxide
| Conditions | Yield |
|---|---|
|
In water; reaction of Na3P with H2O;;
|

silica gel
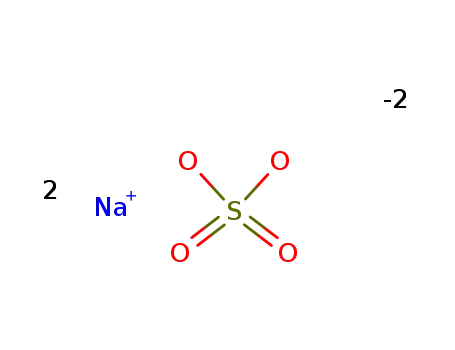
sodium sulfate
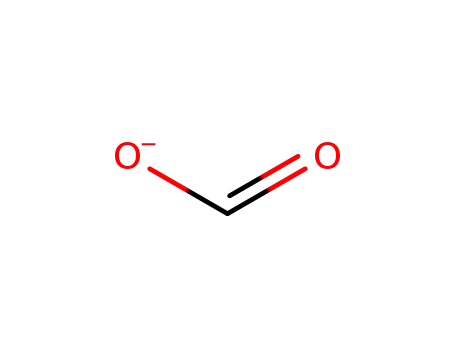
formate

water
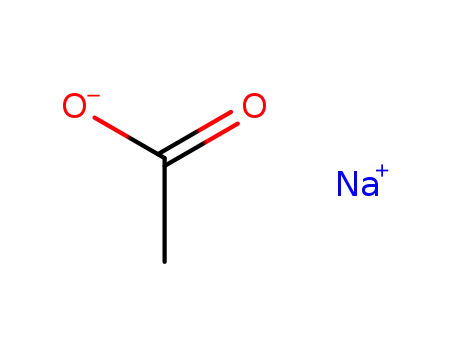
sodium acetate
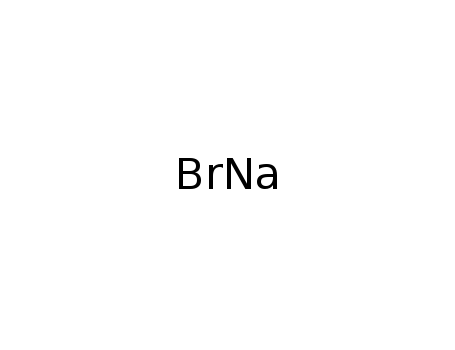
sodium bromide

nickel(II) oxide

nickel
CAS:9005-38-3
CAS:29923-31-7
CAS:156-54-7
CAS:497-76-7