-
- 15383190639
- admin@86-ss.com
Your Location:Home >Products >Organic chemicals >92-69-3

Product Details
-
A Pyrex glass capillary (0.4 mm internal...
Various microwave-heated heterogeneous c...
Palladium nanoparticles stabilized by Pl...
The Pd/Fe3O4 nanocomposite integrates ve...
The present article demonstrates a simpl...
In this article, we report the synthesis...
The synthesis and characterization of a ...
In this work, a novel catalyst Fe@Pd/C w...
[Pd(NH3)4]2+-exchanged sepiolite clay (P...
Four different chitosan-supported pallad...
Herein a novel Pd(II)-polymeric pre-cata...
Different carboxy-functionalized imidazo...
Diazotization reaction, strong exothermi...
We have developed robust, operationally ...
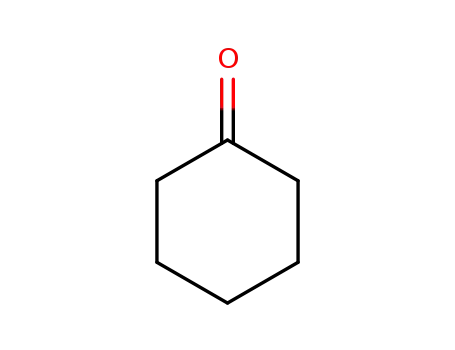
cyclohexanone

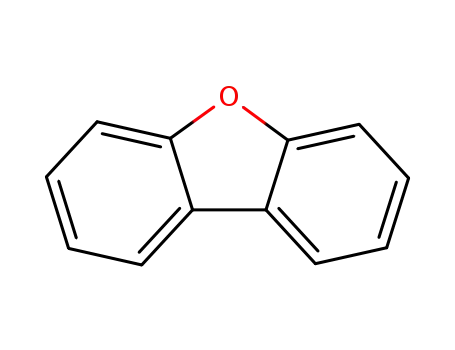
dibenzofuran

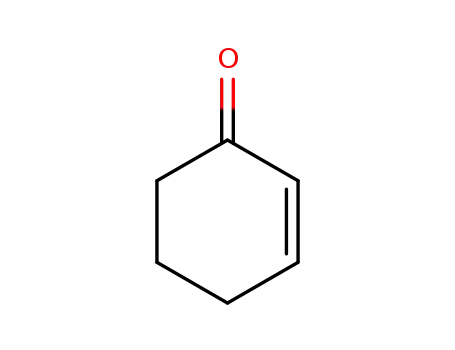
cyclohexenone

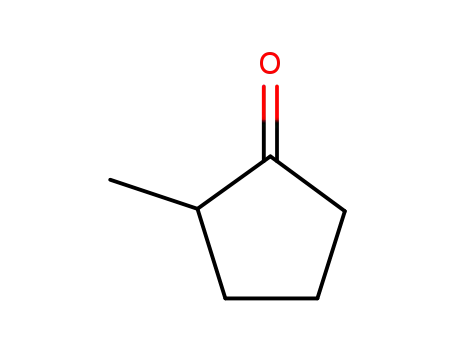
2-Methylcyclopentanone

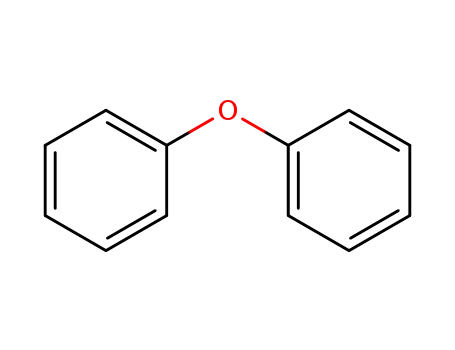
diphenylether

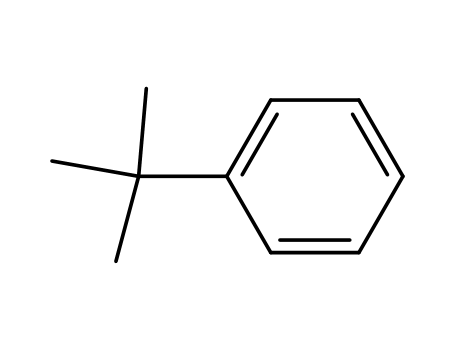
tert-butylbenzene

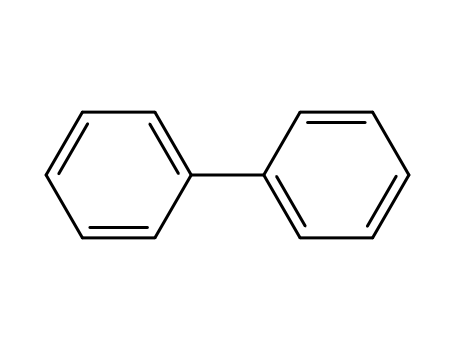
biphenyl

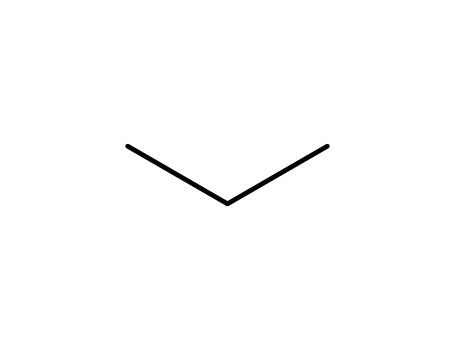
propane

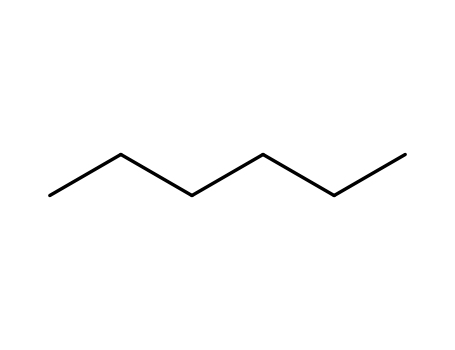
hexane

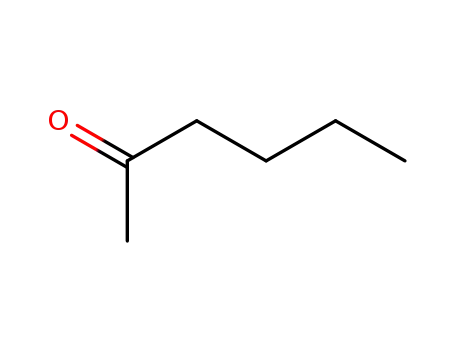
n-hexan-2-one

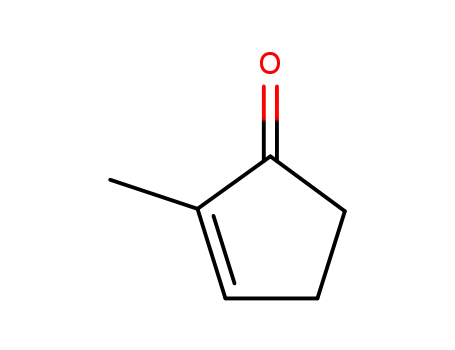
2-methyl-2-cyclopenten-1-one

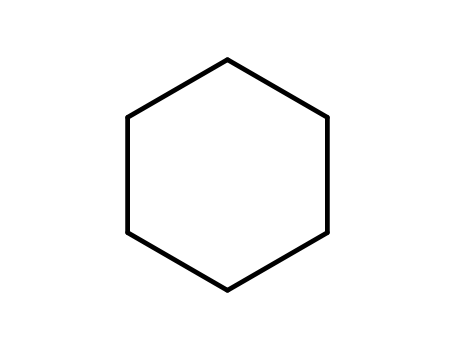
cyclohexane

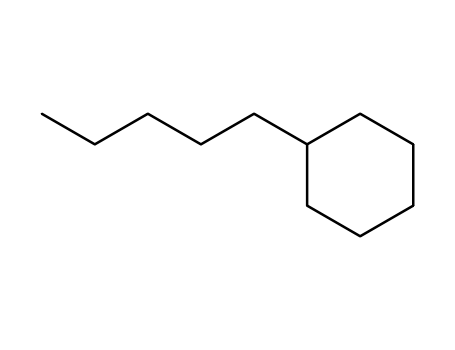
n-pentylcyclohexane

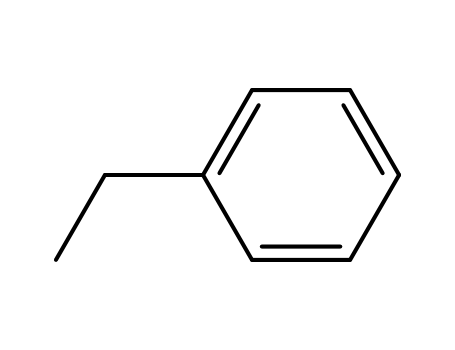
ethylbenzene

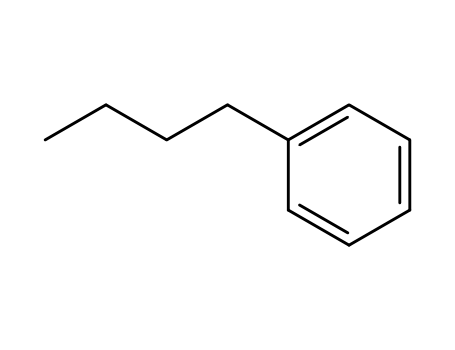
1-butylbenzene

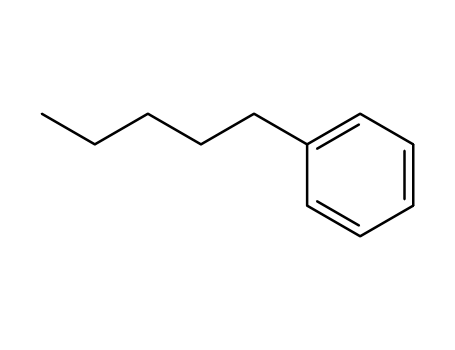
pentylbenzene

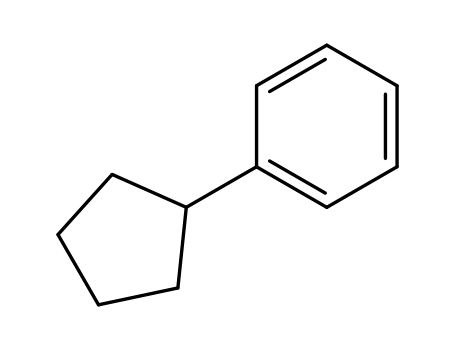
cyclopentylbenzene

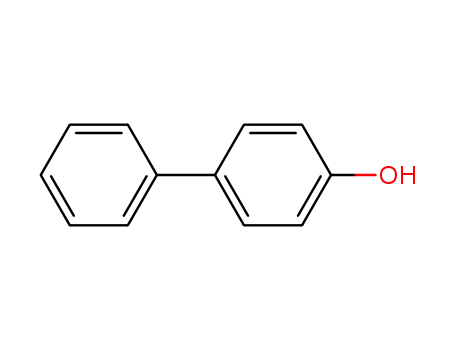
4-Phenylphenol

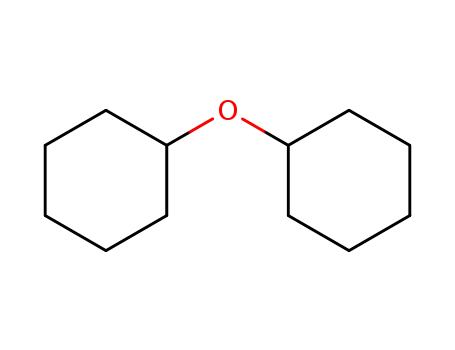
dicyclohexyl ether

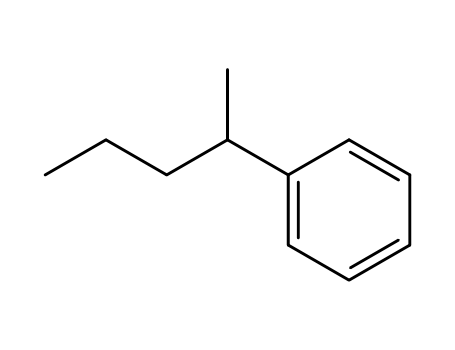
2-phenylpentane

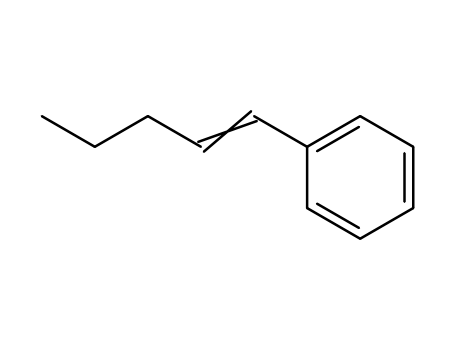
1-pentenylbenzene

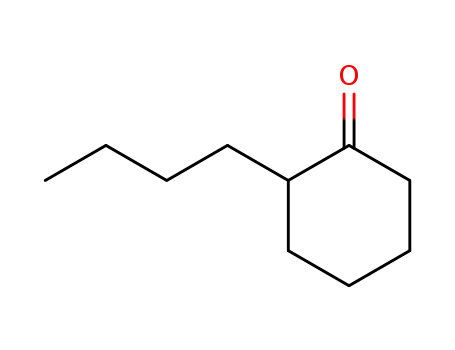
2-butylcyclohexanone

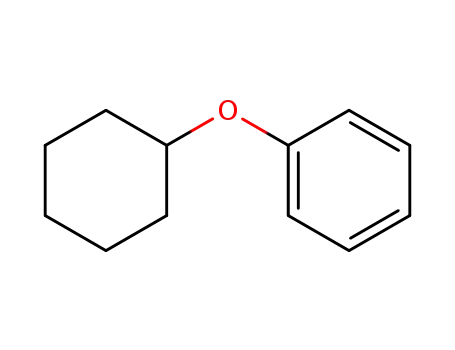
cyclohexylphenyl ether

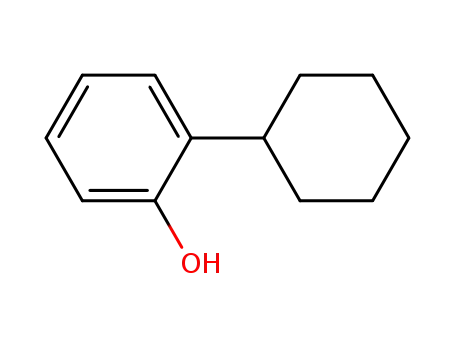
2-cyclohexylphenol

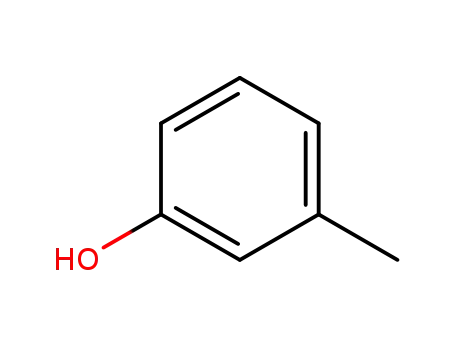
3-methyl-phenol

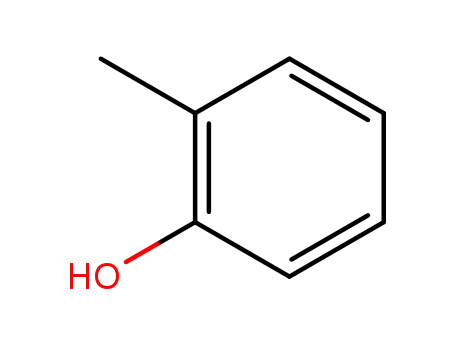
ortho-cresol

2-Phenylphenol

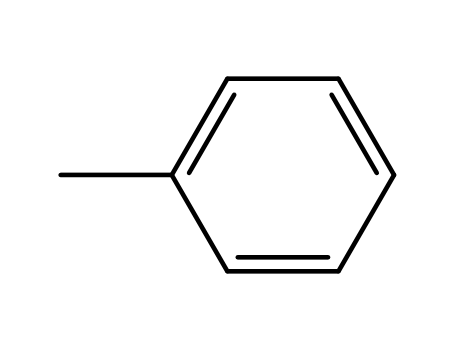
toluene

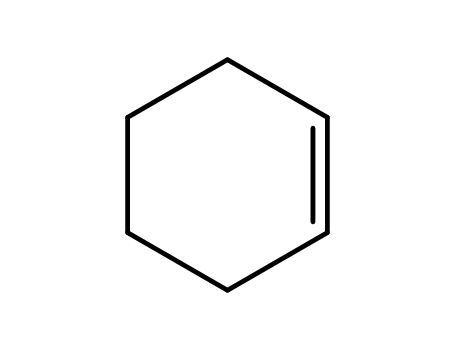
cyclohexene

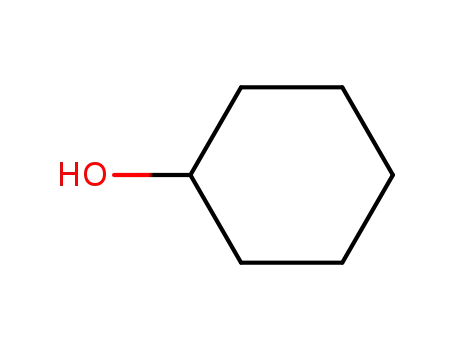
cyclohexanol

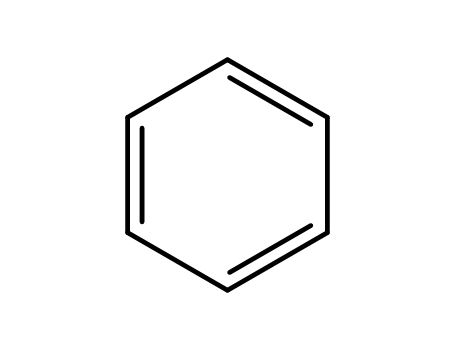
benzene

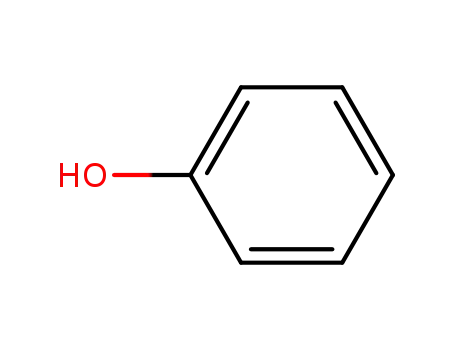
phenol
| Conditions | Yield |
|---|---|
|
With
hydrogen;
1 wtpercent K/1 wtpercent Pt/SiO2;
at 425 ℃;
under 5931.67 Torr;
|

cyclohexanone


dibenzofuran


cyclohexenone


2-Methylcyclopentanone


diphenylether


tert-butylbenzene


biphenyl


propane


hexane


cyclohexane


ethylbenzene


pentylbenzene


cyclopentylbenzene


4-Phenylphenol


dicyclohexyl ether


cyclohexylphenyl ether


2-cyclohexylphenol


ortho-cresol

2-Phenylphenol


toluene


cyclohexene


cyclohexanol


benzene


phenol
| Conditions | Yield |
|---|---|
|
With
hydrogen;
1 wtpercent K/1 wtpercent Pt/SiO2;
at 425 ℃;
under 5931.67 Torr;
|
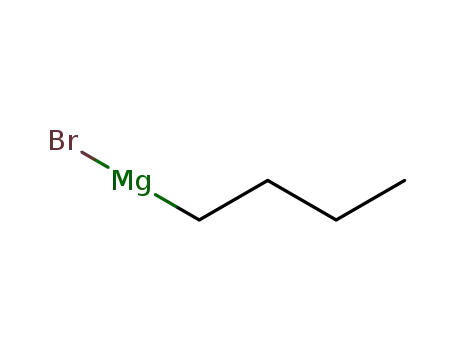
n-butyl magnesium bromide
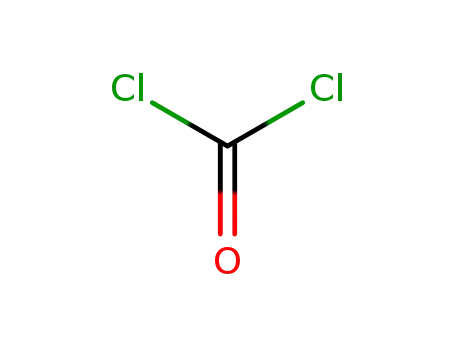
phosgene
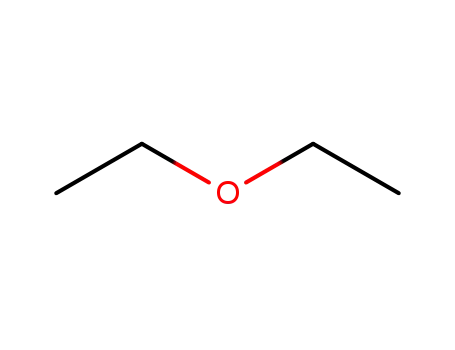
diethyl ether
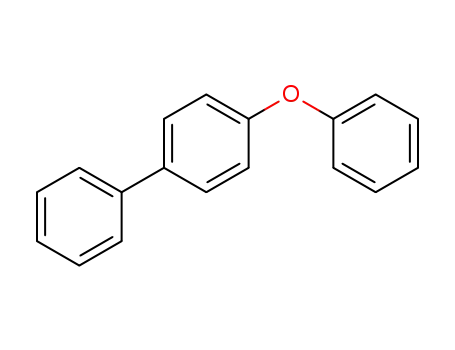
4-Phenoxybiphenyl
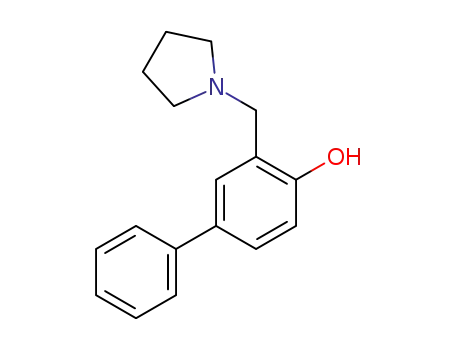
3-Pyrrolidinomethyl-biphenyl-4-ol
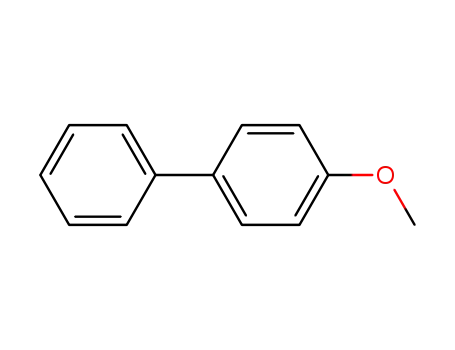
4-methoxylbiphenyl
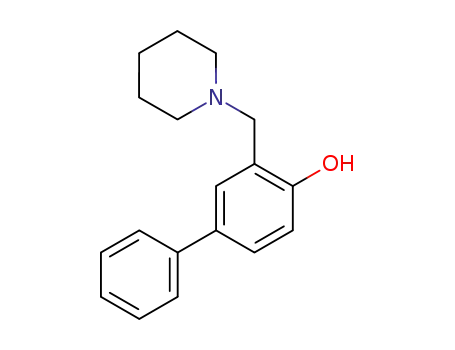
3-Piperidinomethyl-biphenyl-4-ol
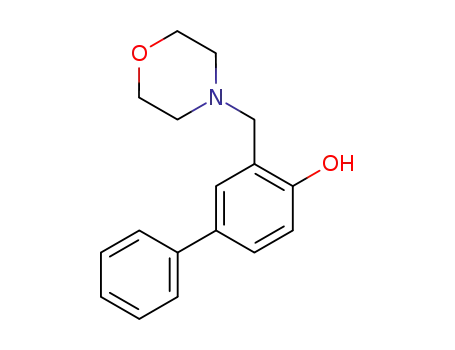
2-Morpholinomethyl-4-phenylphenol
CAS:3081-61-6
CAS:85070-48-0
CAS:533-31-3
CAS:136-52-7