-
- 15383190639
- admin@86-ss.com
Your Location:Home >Products >Organic chemicals >998-40-3


Product Details
|
Synthesis Reference(s) |
Tetrahedron Letters, 25, p. 2493, 1984 DOI: 10.1016/S0040-4039(01)81213-2 |
|
Air & Water Reactions |
Highly flammable. May ignite on contact with air or moist air. Insoluble in water. |
|
Reactivity Profile |
Organophosphates, such as Tributylphosphane, are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides. |
|
Purification Methods |
Fractionally distil it under reduced pressure in an inert atmosphere (N2) through an 8inch gauze-packed column (b 110-111o/10mm) and redistil it in a vacuum, then seal it in thin glass ampoules. It is easily oxidised by air to tri-n-butylphosphine oxide, b 293-296o/745mm. It has a characteristic odour, it is soluble in EtOH, Et2O, and *C6H6 but is insoluble in H2O and less easily oxidised by air than the lower molecular weight phosphines. It forms complexes, e.g. with CS2 (1:1) m 65.5o (from EtOH). [Davies & Jones J Chem Soc 33 1929, Chernick & Skinner J Chem Soc 1401 1956, Beilstein 4 IV 3436.] |
|
General Description |
Tributylphosphane is a colorless to yellowish liquid with a strong garlic-like odor. Tributylphosphane is insoluble in water. Tributylphosphane is liable to heat and ignite spontaneously in air. If involved in a fire phosphine gas, a highly flammable and toxic gas, will evolve. Tributylphosphane is irritating to mucous membranes. |
InChI:InChI=1/C12H27P/c1-4-7-10-13(11-8-5-2)12-9-6-3/h4-12H2,1-3H3
The irreversible addition of alkyl radic...
The dinuclear phosphido-bridged manganes...
The compound Se2 (1) reacts with primary...
The mechanism of the reduction of phosph...
Reduction of phosphine oxides into the c...
Investigations on the boundaries between...
By introducing trimethylsilyl chloride (...
The kinetics of quinuclidine displacemen...
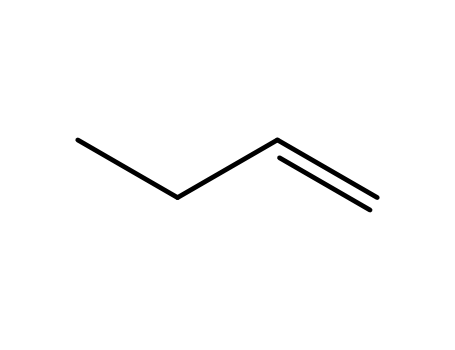
1-butylene

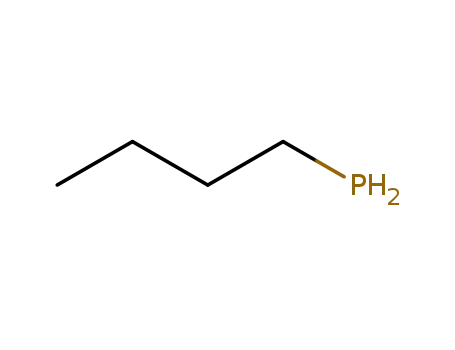
n-butylphosphine


di-n-butylphosphine


tributylphosphine
| Conditions | Yield |
|---|---|
|
at 25 ℃;
UV-Licht.Irradiation;
|

1-butylene

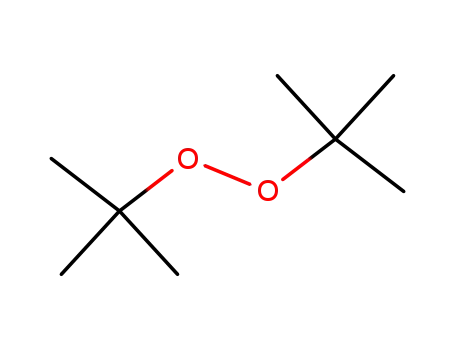
di-tert-butyl peroxide


n-butylphosphine


di-n-butylphosphine


tributylphosphine
| Conditions | Yield |
|---|---|
|
at 122 ℃;
|

1-butylene

di-tert-butyl peroxide
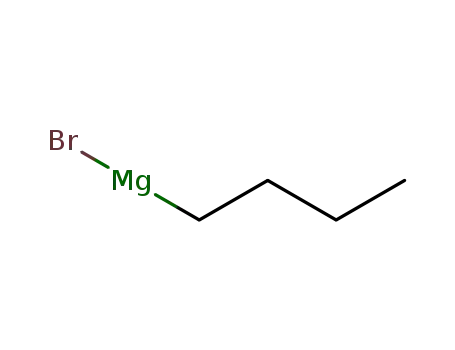
n-butyl magnesium bromide
diethyl ether

tetra-n-butylphosphonium iodide
4-methyl-N-(tri-N-butylphosphoranylidene)-benzenesulfonamide
Tributylphosphine oxide
tributyl-phosphine; compound with gold(I)-chloride
CAS:53-84-9
CAS:5994-61-6
CAS:51446-62-9
CAS:16919-27-0