-
- 15383190639
- admin@86-ss.com
Your Location:Home >Products >Organic chemicals >1310-65-2


Product Details
We propose and demonstrate the direct ap...
The novell ReIII oxo alkyne complexes (η...
The oxidation behaviors of LiH under a h...
Dehydration of CeCl3(H2O)7 following sta...
Behavior of lithium peroxide samples at ...
The enthalpies of solution of Li2ZrO3, L...
The oxyhalide-based solid electrolyte Li...
The parasitic reactions associated with ...
Lithium hydroxide monohydrate based ther...
Through single-step solid-state reaction...
Lithium hydride (LiH) will efficiently r...
The difference methods of neutron diffra...
Hydrogen production via thermochemical w...
Novel bichalcogenides with the general c...
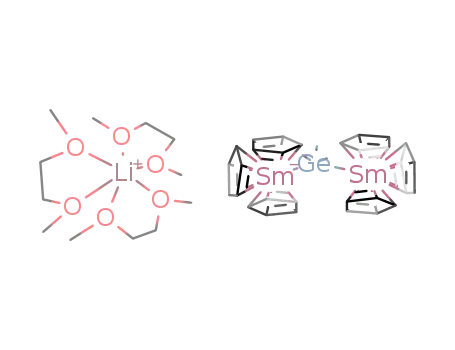
tris(dimethoxyethane-O,O')lithium bis{tris(cyclopentadienyl)samarium}trimethylgermane

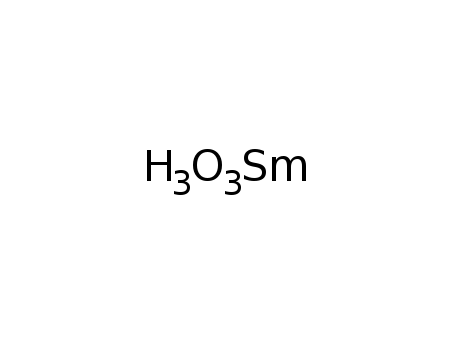
samarium(III) hydroxide

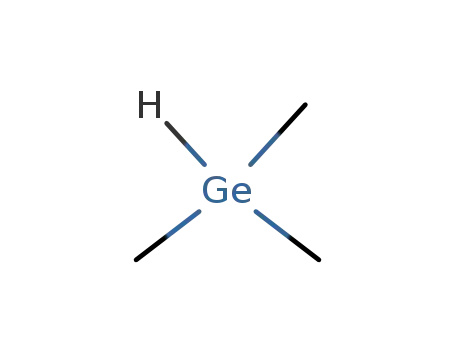
trimethylgermane

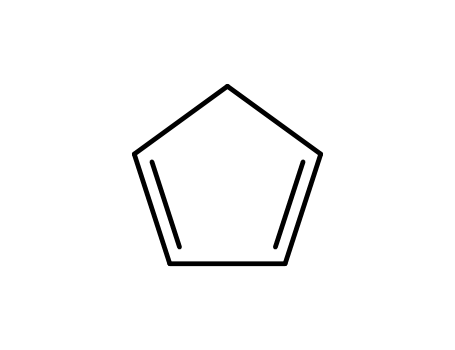
cyclopenta-1,3-diene


lithium hydroxide
| Conditions | Yield |
|---|---|
|
hydrolysis;
|
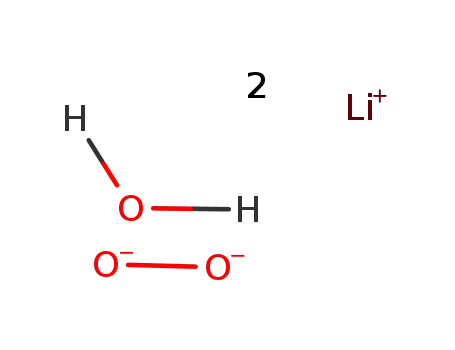
lithium peroxide monohydrate

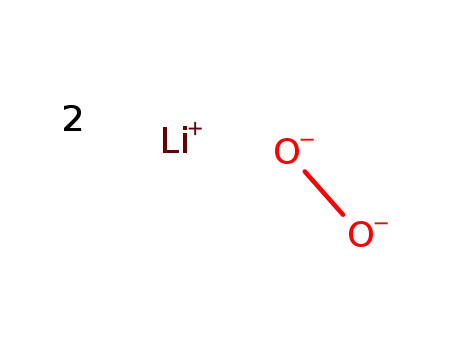
lithium peroxide

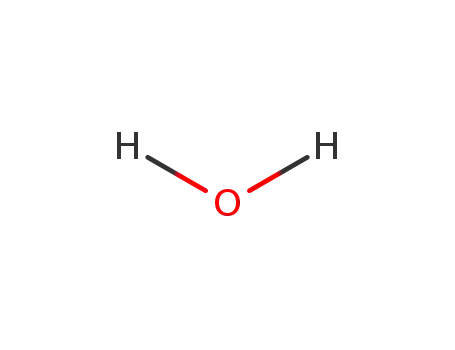
water

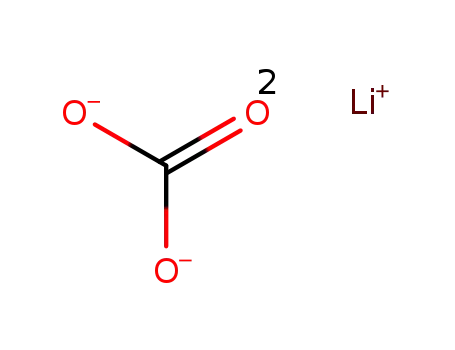
lithium carbonate


lithium hydroxide
| Conditions | Yield |
|---|---|
|
In
neat (no solvent);
heating in air to 25°C-340°C at the relative humidity 96% for 1 h; X-ray phase analysis;
|

water

lithium
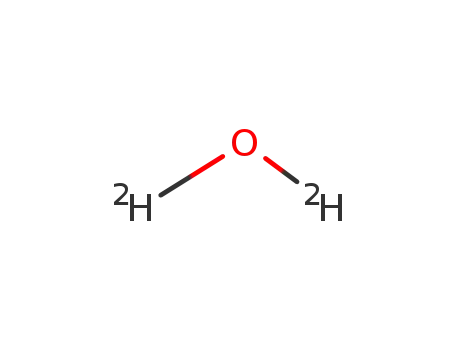
water-d2
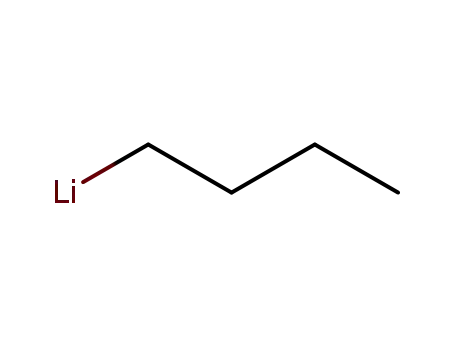
n-butyllithium
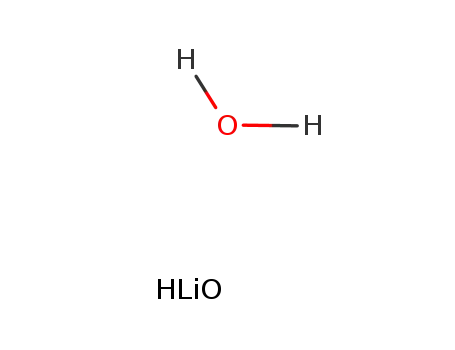
lithium hydroxide monohydrate

lithium bromide

lithium carbonate
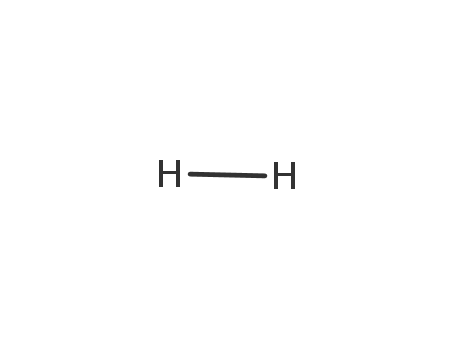
hydrogen
CAS:9003-98-9
CAS:2023788-19-2
CAS:475-83-2