-
- 15383190639
- admin@86-ss.com
Your Location:Home >Products >Organic chemicals >72-18-4
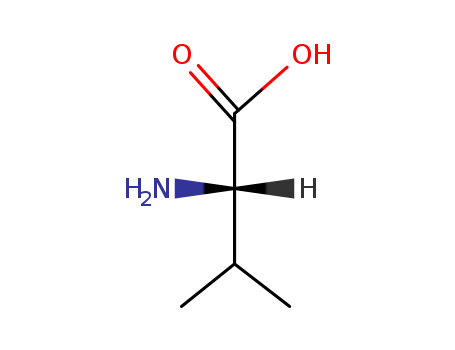

Product Details
|
Industrial Applications |
Description: Valine (L-valine) is one of the three branched-chain amino acids (BCAAs) widely applied in various industrial fields such as pharmaceuticals, cosmetics, food, and feed. |
|
Biological Importance |
Role in Muscle Tissue Recovery and Repair: Valine aids in muscle tissue recovery and repair, as well as increasing energy and endurance. |
|
Medical and Nutritional Uses |
Inhibition of Tissue Protein Degradation: Valine can inhibit the degradation of tissue proteins, improving the carcass quality of livestock and poultry. |
|
Production Methods |
Microbial Fermentation: Currently, microbial fermentation is the primary method for producing L-valine, with mutagenesis and metabolic engineering strategies employed to enhance production. |
|
General Description |
L-Valine is one of 20 proteinogenic amino acids essential for human health, often utilized in the biosynthesis of proteins. This amino acid is classified as branched-chain because its molecular structure includes a side chain. It is not synthesized in animals, and thus it must be obtained through diet. L-Valine is well known for its benefits in muscle growth and tissue repair, energy provision, and immune function enhancement. Foods high in L-Valine include grains, dairy, mushrooms, peanuts, soy, and meat. In industries, it’s used in the formulation of fitness supplements and medicines due to its effects on muscle metabolism and growth. Yields of L-valine are also used as a feed additive to support growth in farm animals. |
InChI:InChI:1S/C5H11NO2/c1-3(2)4(6)5(7)8/h3-4H,6H2,1-2H3,(H,7,8)
Surfactins are natural biosurfactants wi...
Carboxypeptidases enzymatically cleave t...
Fungal natural products play a prominent...
Amino acids are key synthetic building b...
C28H41N5O10

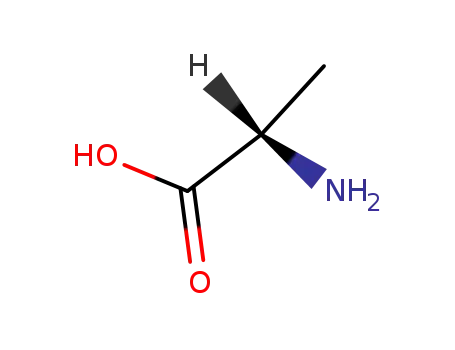
L-alanin

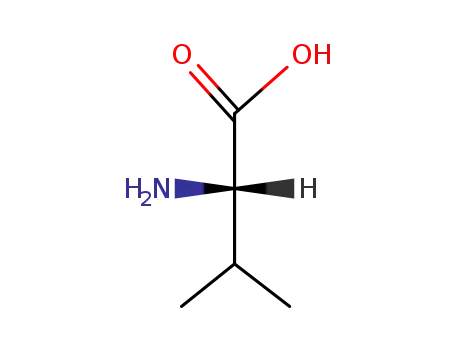
L-valine

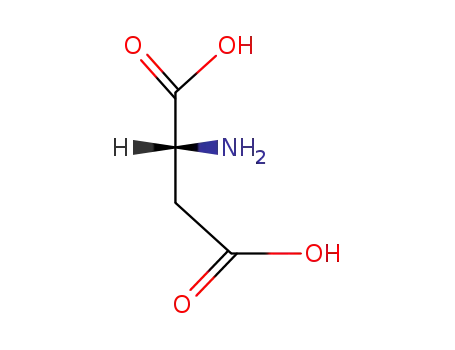
(2R)-aspartic acid
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; water;
at 120 ℃;
for 14h;
|
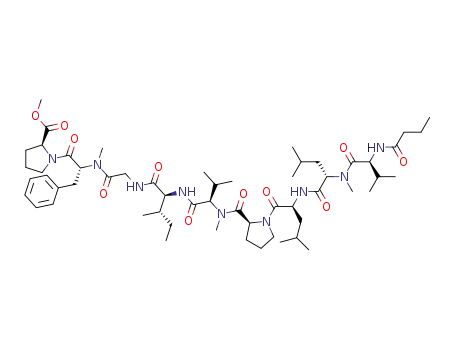
amantamide


L-valine

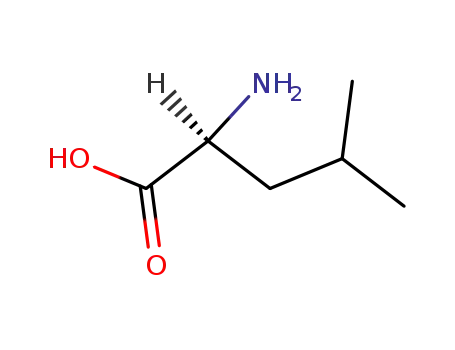
L-leucine

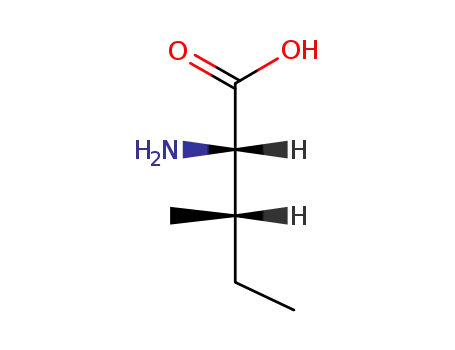
L-isoleucine

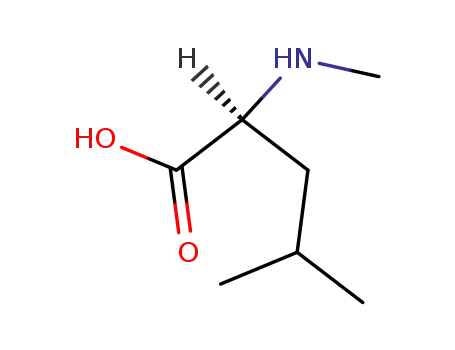
N-methyl-L-leucine

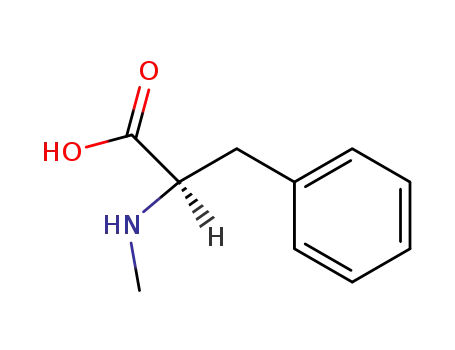
N-methyl-D-phenylalanine

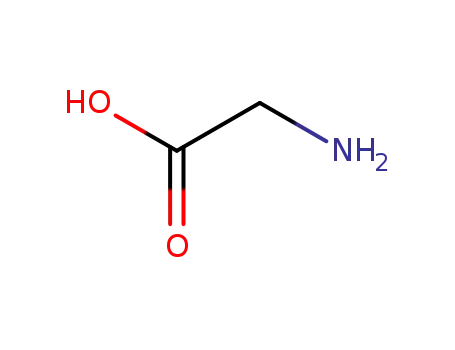
glycine

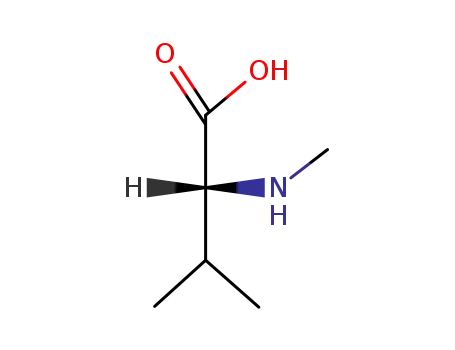
(-)-α-methylamino-β-methylbutyric acid

L-proline
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
water;
at 110 ℃;
for 24h;
|
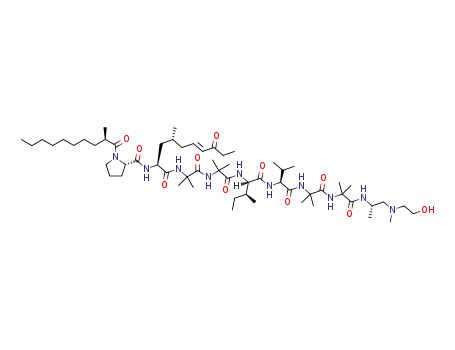
trichoderin A1
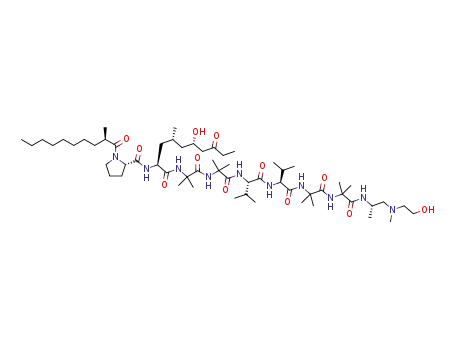
trichoderin B
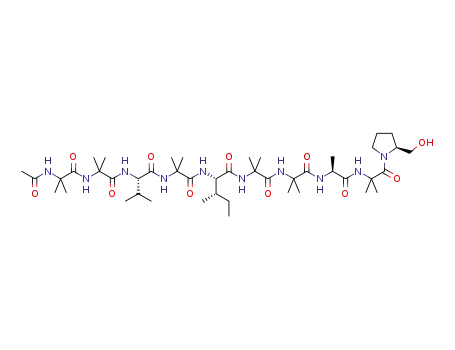
aspereline A
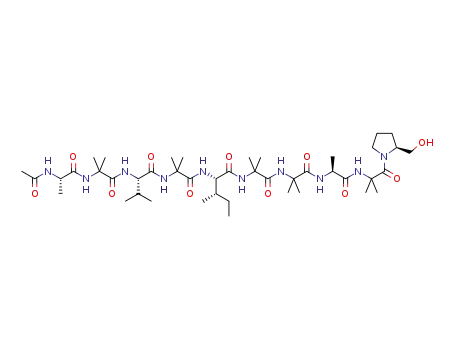
aspereline B
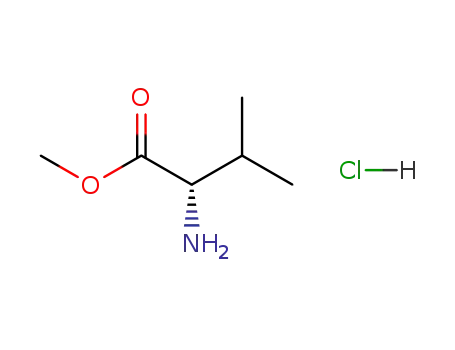
L-valine methylester hydrochloride
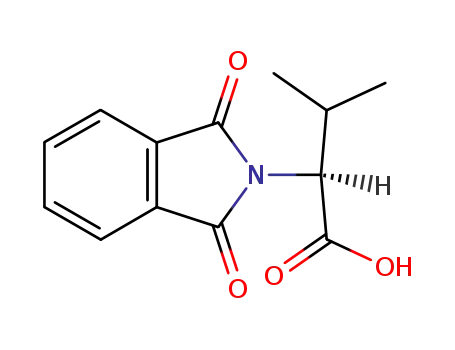
N-phthaloyl L-valine
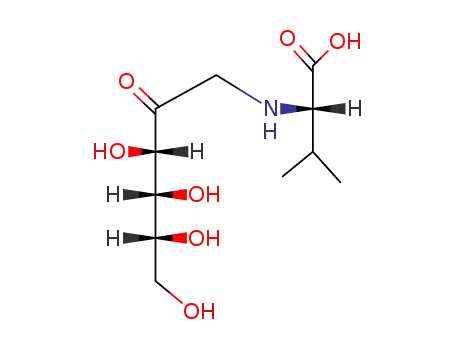
N-(1-deoxy-D-fructos-1-yl)-L-valine
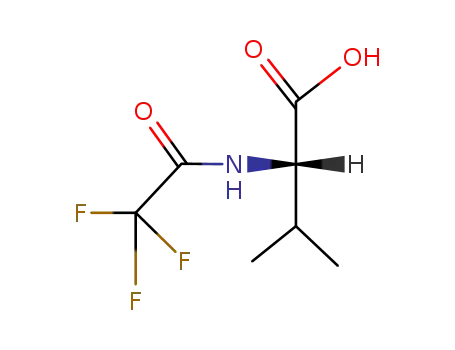
N-trifluoroacetyl-L-valine
CAS:3081-61-6
CAS:195875-84-4
CAS:103-90-2
CAS:70-18-8