-
- 15383190639
- admin@86-ss.com
Your Location:Home >Products >Organic chemicals >123-86-4

Product Details
|
Industrial Application and Production |
Butyl acetate (BuAc) is widely utilized as a solvent in various industries including manufacturing, pharmaceuticals, cosmetics, and food. It is a key component in the production of adhesives, lacquers, coatings, paints, and fruit flavors. Industrial-scale production involves the esterification of n-butanol with acetic acid using strong acids as catalysts, typically solid-acidic catalysts like ion exchange resins. |
|
Health and Safety Concerns |
Butyl acetate is known to be an irritating, flammable, and potentially explosive toxic gas. High concentrations of inhaled butyl acetate can lead to cardiovascular and neurological diseases. Therefore, developing semiconductor sensors with high sensitivity and selectivity for detecting butyl acetate is crucial for preventing accidents and protecting health in industrial settings. |
|
Production Challenges and Alternative Methods |
Traditional production methods suffer from low equilibrium conversion rates and high energy consumption during product separation and purification. Transesterification processes offer higher economic performance compared to esterification. Enzymatic approaches, although more environmentally friendly, incur higher costs due to lipase production. Alternatively, metabolic engineering techniques can be employed for in vivo biosynthesis of n-butyl acetate from biomass, offering a promising alternative route. |
|
Environmental Impact and Waste Management |
Butyl acetate is commonly used as a solvent for drug extraction in pharmaceutical production processes, resulting in wastewater solutions containing high concentrations of BA. Discharging these chemical waste liquids without separation and recovery not only leads to underutilization of resources but also causes serious environmental pollution and economic losses for enterprises. Therefore, efficient separation and recovery methods are essential for mitigating environmental impact and maximizing resource utilization. |
|
Physical properties |
Clear, colorless liquid with a strong fruity odor resembling bananas. Sweetish taste as low concentrations (<30 μg/L). Experimentally determined detection and recognition odor threshold concentrations were 30 μg/m3 (6.3 ppbv) and 18 μg/m3 (38 ppbv), respectively (Hellman and Small, 1974). Cometto-Mu?iz et al. (2000) reported nasal pungency threshold concentrations ranged from approximately 550 to 3,500 ppm. |
|
Definition |
ChEBI: The acetate ester of butanol. |
|
Production Methods |
Butyl alcohol is combined with acetic acid in the presence of a catalyst such as sulfuric acid. After esterification is complete, the solution is distilled to yield butyl acetate . |
|
Preparation |
By esterification of n-butyl alcohol with acetic acid. |
|
Aroma threshold values |
Detection: 10 to 500 ppb |
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 73, p. 5265, 1951 DOI: 10.1021/ja01155a075The Journal of Organic Chemistry, 39, p. 3728, 1974 DOI: 10.1021/jo00939a026 |
|
General Description |
A clear colorless liquid with a fruity odor. Flash point 72 - 88°F. Density 7.4 lb / gal (less than water). Hence floats on water. Vapors heavier than air. |
|
Air & Water Reactions |
Highly flammable. Very slightly soluble in water. |
|
Reactivity Profile |
Butyl acetate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Attacks many plastics. [Handling Chemicals Safely 1980. p. 233]. |
|
Hazard |
Skin irritant, toxic. Flammable, moderate fire risk. Eye and upper respiratory tract irritant. |
|
Health Hazard |
Exposures to n-butyl acetate cause harmful effects that include, but are not limited to, coughing and shortness of breath. High concentrations have a narcotic effect, with symp toms such as sore throat, abdominal pain, nausea, vomiting, and diarrhea. High concen trations of n-butyl acetate cause severe poisoning. Prolonged periods of exposure cause adverse effects to the lungs, the nervous system, and the mucous membranes. Repeated skin contact causes skin dryness or cracking, and dermatitis. |
|
Fire Hazard |
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. |
|
Flammability and Explosibility |
Flammable |
|
Chemical Reactivity |
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. |
|
Biochem/physiol Actions |
Taste at 30 ppm |
|
Safety Profile |
Moderately toxic by intraperitoneal route. Mdly toxic by inhalation and ingestion. An experimental teratogen. A skin and severe eye irritant. Human systemic effects by inhalation: conjunctiva irritation, unspecified nasal and respiratory system effects. A mild allergen. High concentrations are irritating to eyes and respiratory tract and cause narcosis. Evidence of chronic systemic toxicity is inconclusive. Flammable liquid. Moderately explosive when exposed to flame. Ignites on contact with potassium tert-butoxide. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid and irritating fumes. See also ESTERS. |
|
Carcinogenicity |
There are no indications of mutagenic or cytogenic effects for n-butyl acetate. |
|
Source |
Identified as a volatile constituent released by fresh coffee beans (Coffea canephora variety Robusta) at different stages of ripeness (Mathieu et al., 1998). Also identified among 139 volatile compounds identified in cantaloupe (Cucumis melo var. reticulates cv. Sol Real) using an automated rapid headspace solid phase microextraction method (Beaulieu and Grimm, 2001). |
|
Environmental fate |
Biological. Heukelekian and Rand (1955) reported a 5-d BOD value of 0.52 g/g which is 23.5% of the ThOD value of 2.21 g/g. Photolytic. Butyl acetate reacts with OH radicals in the atmosphere at a rate constant of 4.15 x 10-12 cm3/molecule?sec at 296 K (Wallington et al., 1988b). Chemical/Physical. Hydrolyzes in water forming 1-butanol and acetic acid. Estimated hydrolysis half-lives at 20 °C: 11.4 d at pH 9.0, 114 d at pH 8.0, and 3.1 yr at pH 7.0 (Mabey and Mill, 1978). At an influent concentration of 1,000 mg/L, treatment with GAC resulted in an effluent concentration of 154 mg/L. The adsorbability of the carbon was 169 mg/g carbon (Guisti et al., 1974). |
|
storage |
n-Butyl acetate should be kept stored in a segregated and approved area. Workers should keep the container in a cool, well-ventilated area, closed tightly, and sealed until ready for use. Workers should avoid all possible sources of ignition/spark at the workplace |
|
Purification Methods |
Distil, reflux with successive small portions of KMnO4 until the colour persists, dry with anhydrous CaSO4, filter and redistil. [Beilstein 2 IV 143.] |
|
Waste Disposal |
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. |
|
Precautions |
On exposure to Butyl acetate, immediately wash with plenty of water, also under the eyelids, for at least 15 min. Remove contact lenses. Butyl acetate?is flammable in the pres ence of open flames, sparks, oxidizing materials, acids, and alkalis. It poses explosion risk in the presence of mechanical impact. For health safety, management authorities should provide exhaust ventilation facilities at the workplace to keep the airborne concentrations of vapors of Butyl acetate?below TLV. |
InChI:InChI=1/C6H12O2/c1-3-4-5-8-6(2)7/h3-5H2,1-2H3
HTiNbO5, HNbMoO6·H2O and HNbWO 6·1.5H2O ...
This paper reports the facile synthesis ...
Catalysts prepared by reacting polyethyl...
An acid activated montmorillonite, treat...
In this work, maize starch (MS) was succ...
Esterification of acetic acid with n-But...
Crosslinked sulphonated polystyrene (Dow...
A novel carbon-based strong acid catalys...
Cycloaddition reactions of labelled 6-me...
Aiming at inexpensive Bronsted-acidic io...
In this contribution we report a synthet...
A novel fibrous sulfated ZrO2 (SO4 2-/Zr...
A novel solid superacid catalyst TiO2-Zr...
Graphene with its two-dimensional sheet ...
Candida rugosa lipase (CRL) and porcine ...
Esterification of sodium salt of carboxi...
The aim of this study was to analyze the...
Butyl acetate, a renewable biofuel addit...
The paper describes the preparation of a...
-
A series of Ni-based catalysts were prep...
The novel biacidic carbon has been synth...
The transesterification of methyl, aceta...
In this work, the inclusion of free sulf...
Pyridinium hydrogen sulfate ionic liquid...
An enhanced small-scale reaction calorim...
Zinc oxide and its composites with Hβ ze...
Crosslinked poly(4-vinylpyridine) hydroc...
Magnetically active and SO3H-functionali...
A tubular hydroxy sodalite (SOD) membran...
Full details of a high yielding synthesi...
The influence of low-frequency ultrasoun...
The use of an near-IR fiber-optic spectr...
The liquid-phase esterification of aceti...
Trimethylhydroquinone-1-monoacetate (TMH...
Diffusion-ordered NMR spectroscopy (DOSY...
-
A method of synthesizing stable and reac...
Sulfonic acid-functionalized hybrid sili...
A series of tungstate promoted β-SiC cat...
A series of oxazadisilinane compounds we...
This work reports the performance of a n...
In this study, the reaction equilibrium ...
Two γ-alumina-supported molybdenum oxide...
The phase behavior (KT) and the equilibr...
A new spinel-style S2O82-/ZnAl2O4 solid ...
Three natural basaltic samples were coll...
New types of mesoporous SA/MCM-41 solid ...
We report the dehydrogenative synthesis ...
The Vilsmeier reagent (VR), first report...
Geminal diacetates have been used as sus...
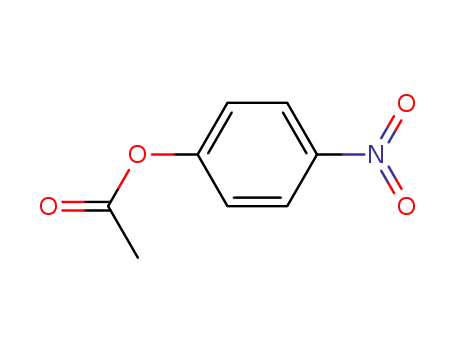
4-nitrophenol acetate

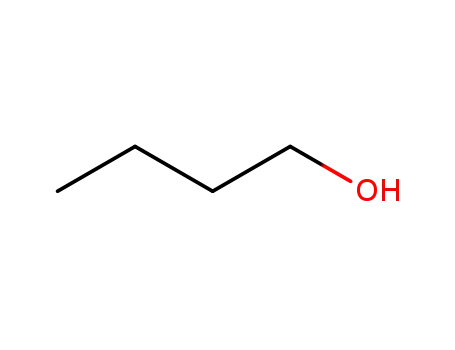
butan-1-ol

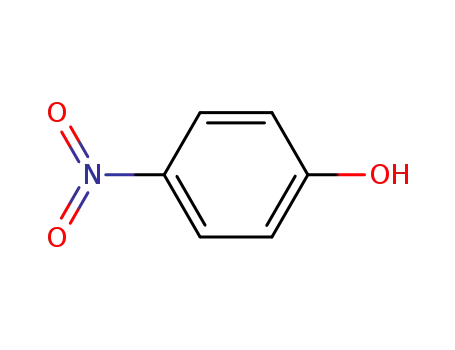
4-nitro-phenol

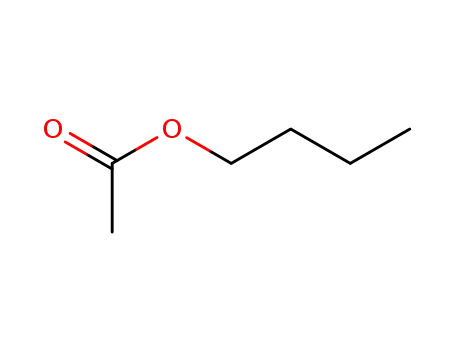
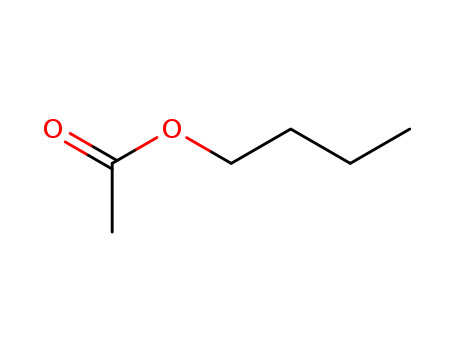
acetic acid butyl ester
| Conditions | Yield |
|---|---|
|
With
1H-imidazole; benzoic acid;
In
chloroform;
for 24h;
Reflux;
|
49 %Chromat. |
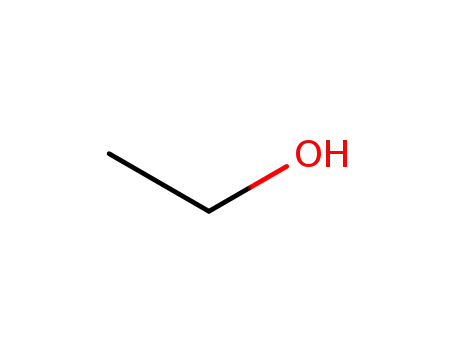
ethanol

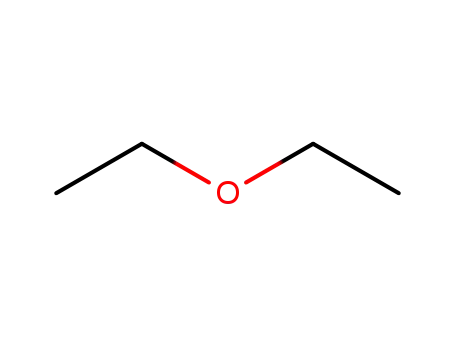
diethyl ether

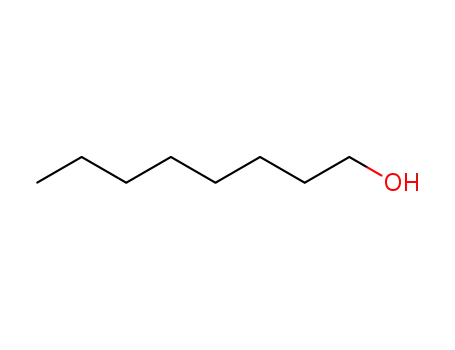
octanol

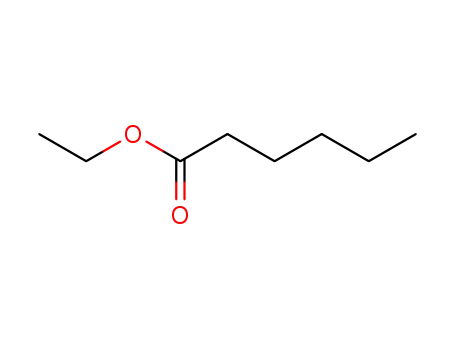
Ethyl hexanoate

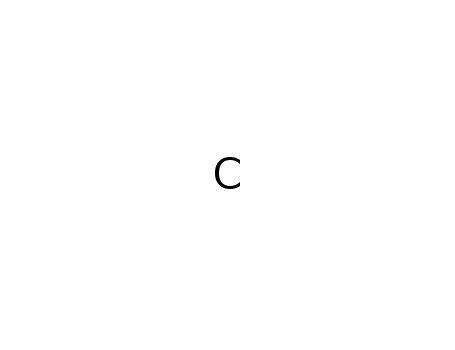
methane

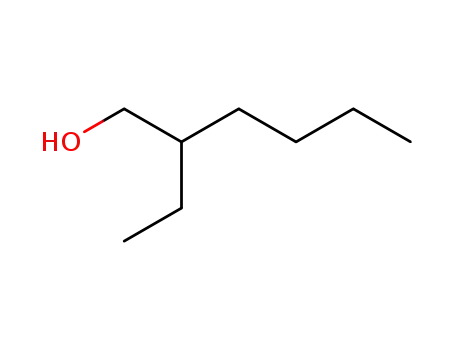
2-Ethylhexyl alcohol

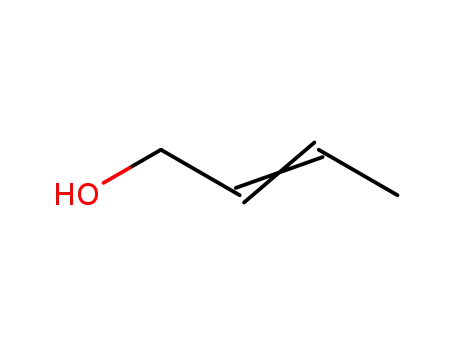
(E/Z)-2-buten-1-ol

ethene

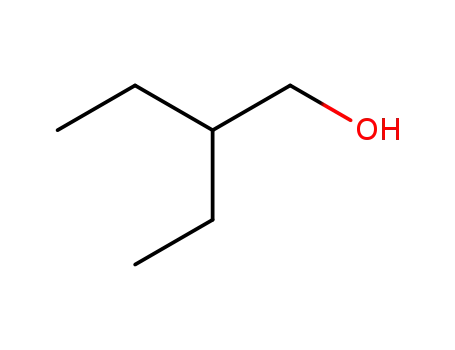
2-ethyl-1-butanol

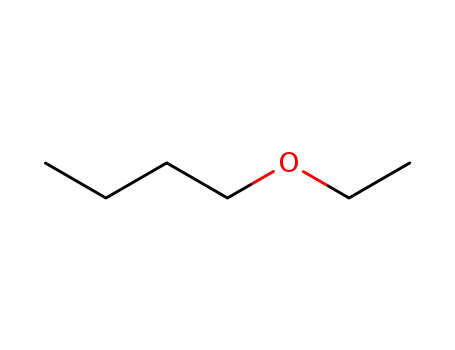
butyl ethyl ether

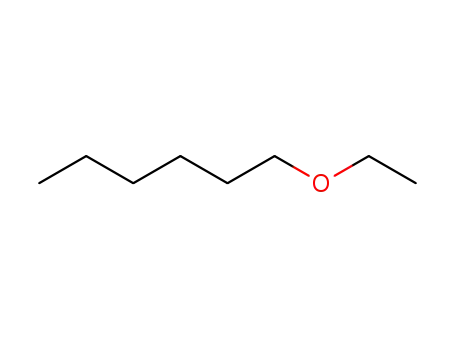
ethyl n-hexyl ether


acetic acid butyl ester

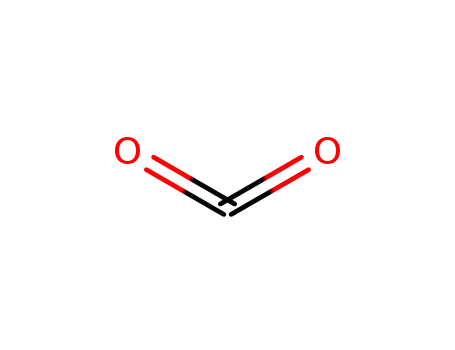
carbon dioxide

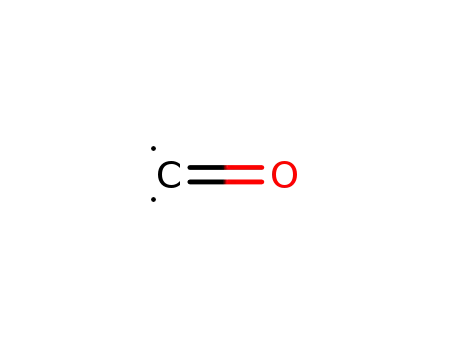
carbon monoxide

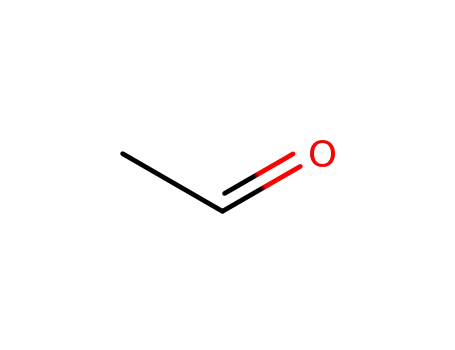
acetaldehyde

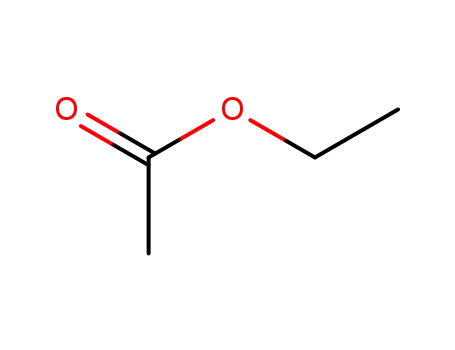
ethyl acetate

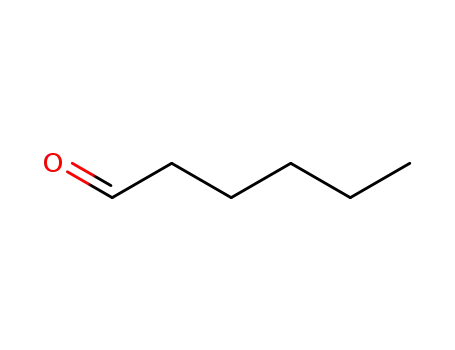
hexanal

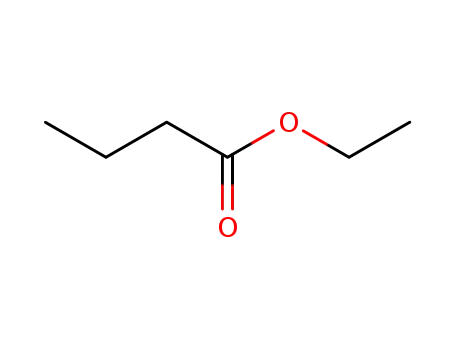
butanoic acid ethyl ester

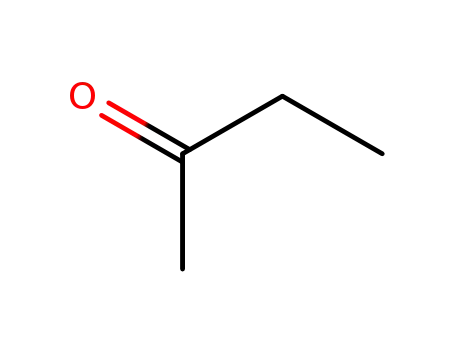
butanone

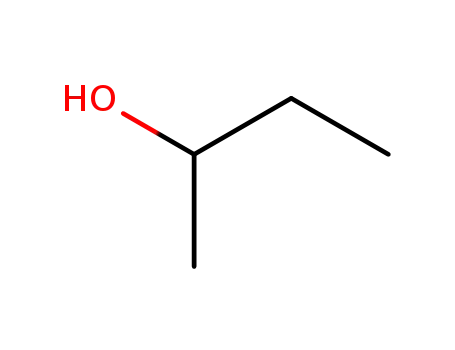
iso-butanol

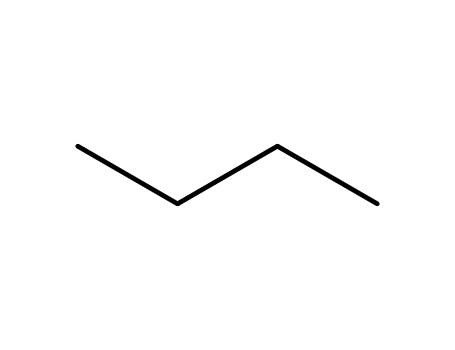
n-butane


butan-1-ol

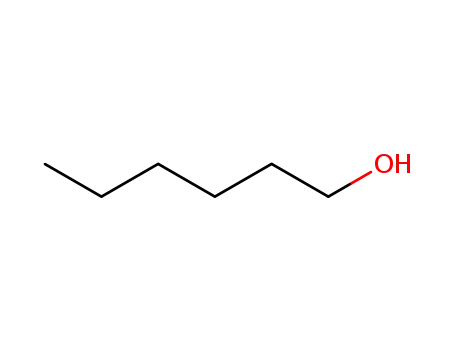
hexan-1-ol
| Conditions | Yield |
|---|---|
|
at 295 ℃;
Autoclave;
Supercritical conditions;
|
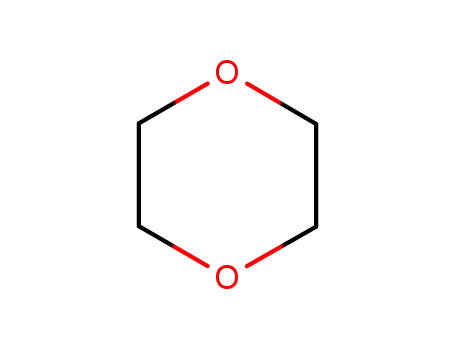
1,4-dioxane
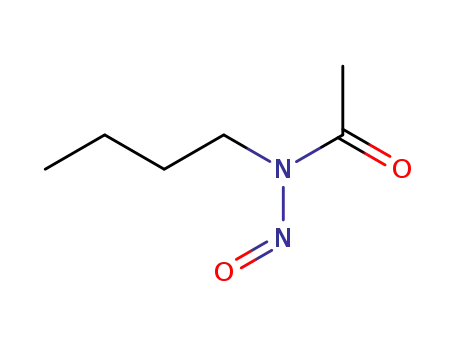
N-butyl-N-nitroso-acetamide
tetrachloromethane
Acetyl bromide
acrylic acid n-butyl ester
3-methylthio-4-phenyl-1,2-dithiolium iodide
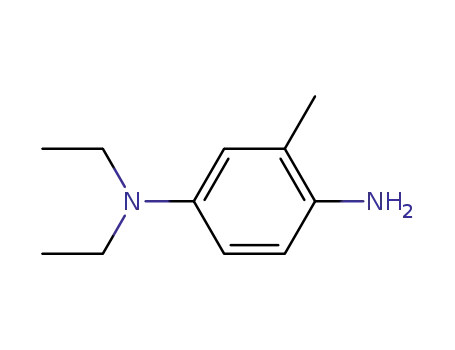
4-diethylamino-o-toluidine
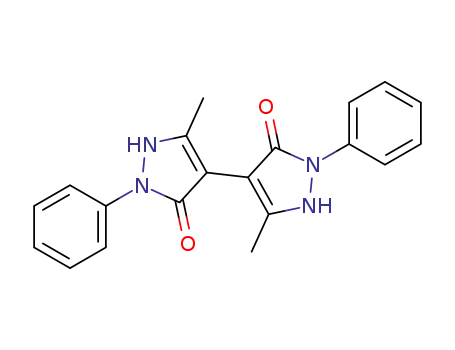
5,5'-dimethyl-2,2'-diphenyl-1,2,1',2'-tetrahydro-[4,4']bipyrazolyl-3,3'-dione
CAS:3081-61-6
CAS:85070-48-0
CAS:9005-38-3
CAS:201530-41-8