-
- 15383190639
- admin@86-ss.com
Your Location:Home >Products >Pharmaceutical intermediate >499-44-5

Product Details
|
benefits |
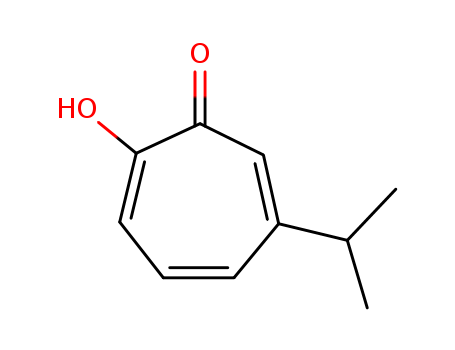
Hinokitiol (β-thujaplicin) is a naturally occurring antioxidant, found in the heartwood of certain plants. It acts as a metal chelator and, also, enhances the activity of superoxide dismutase (Huang et al., 2015). It was found to be very protective in the assay. It has not been previously studied as a HC protectant. In addition to its antioxidant properties, hinokitiol has been shown to reduce inflammation via suppression of NFκB, and metalloproteinases, and to activate caspase 3. The former activities could contribute to its protective effect.Hinokitiol is a superpower ingredient that has anti-inflammatory, antioxidant, antibacterial, anti-fungal and anti-melanogenic properties. Best of all, hinokitiol is as gentle as it is powerful. Hinokitiol’s properties allow it to target the inflammatory redness and blemishes seen in rosacea and acne. Hinokitiol is effective against P. acnes bacteria, and there is no known acquired resistance to it, unlike other prescription antibiotics. |
|
Safety |
The safety of hinokitiol has been tested in rats and no carcinogenic effect to rats was found.In 2006, hinokitiol was categorized under the Domestic substances list (DSL) in Canada as non-persistent, non-bioaccumulative and non-toxic to aquatic organisms. |
|
Anticancer Research |
Studied in xenograft tumors such lung adenocarcinoma cell, EGFR-TKI-resistantlines PC9-IR and H1975 in which the growth inhibition was observed, a novel antitumormechanism was hypothesized. In summary, the hinokitiol can induce DNAdamage and autophagy (Rodrigues et al. 2015). |
|
Definition |
ChEBI: A monoterpenoid that is cyclohepta-2,4,6-trien-1-one substituted by a hydroxy group at position 2 and an isopropyl group at position 4. Isolated from Thuja plicata and Chamaecyparis obtusa, it exhibits antimicrobial activities. |
InChI:InChI=1/C10H12O2/c1-7(2)8-4-3-5-9(11)10(12)6-8/h3-7H,1-2H3,(H,11,12)
The invention discloses a method for pre...
The invention discloses a method for pre...
The invention belongs to the technical f...
The invention belongs to the technical f...
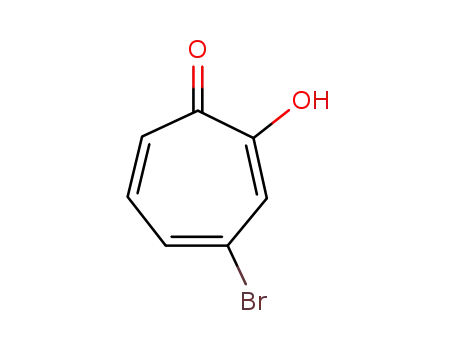
4-bromo-2-hydroxycycklohepta-2,4,6-trien-1-one

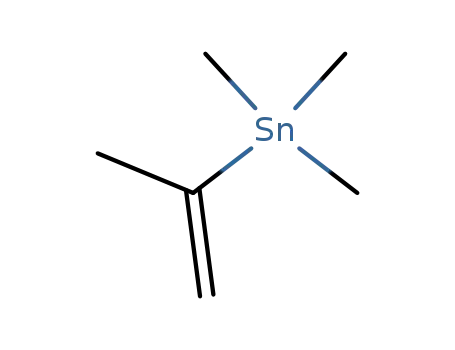
isopropenyltrimethylstannane

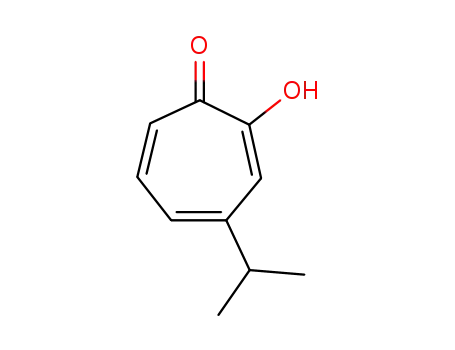
hinokitiol
| Conditions | Yield |
|---|---|
|
4-bromo-2-hydroxycycklohepta-2,4,6-trien-1-one; isopropenyltrimethylstannane;
With
bis-triphenylphosphine-palladium(II) chloride;
In
1,4-dioxane;
for 1h;
Reflux;
With
5%-palladium/activated carbon; hydrogen;
In
ethanol;
at 20 ℃;
for 24h;
under 760.051 Torr;
|
53% |
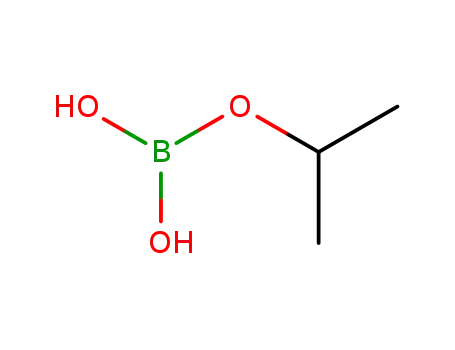
isopropoxyboronic acid

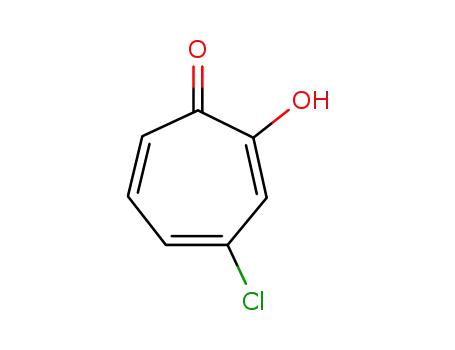
2-hydroxy-4-chloro-2,4,6-cycloheptatrien-1-one


hinokitiol
| Conditions | Yield |
|---|---|
|
With
potassium phosphate;
In
tetrahydrofuran;
at 100 ℃;
for 2h;
|
97.5% |
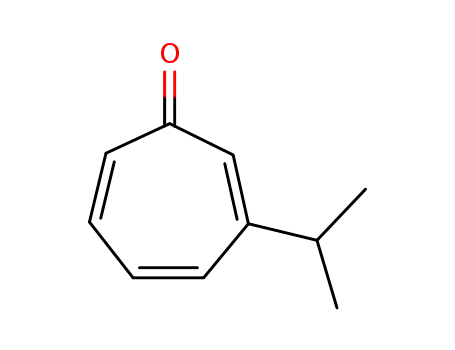
β-isopropyltropolone
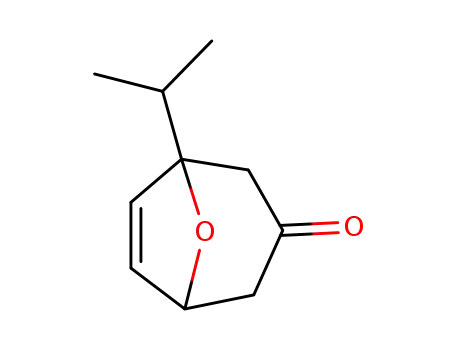
1-isopropyl-8-oxa-bicyclo[3.2.1]oct-6-en-3-one
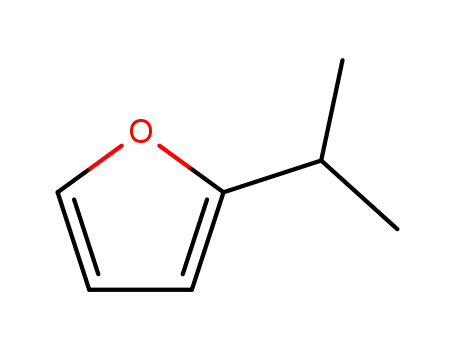
2-isopropylfuran
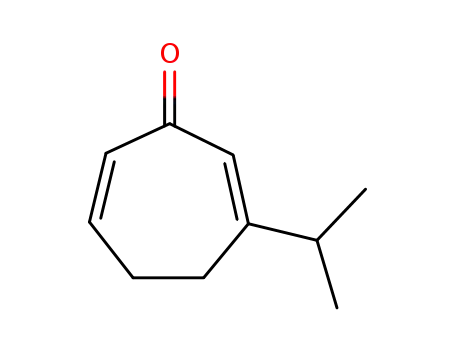
3-Isopropylcyclohepta-2,6-dienon
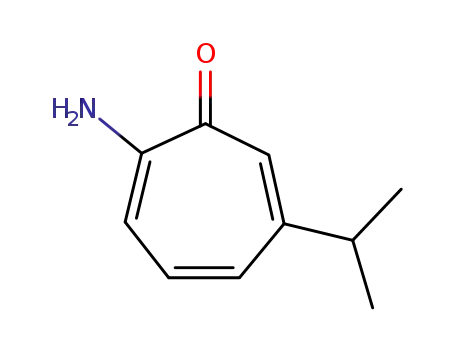
2-amino-6-isopropyl-cycloheptatrienone
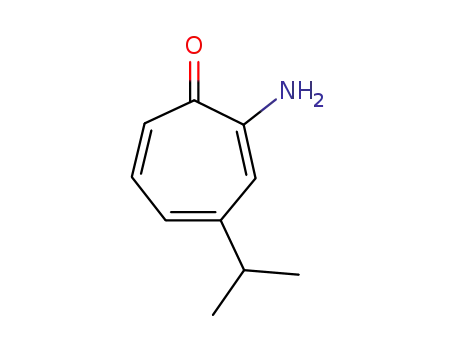
2-amino-4-isopropyl-cycloheptatrienone
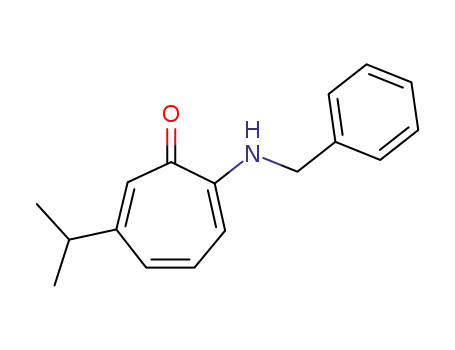
2-benzylamino-6-isopropyl-cycloheptatrienone
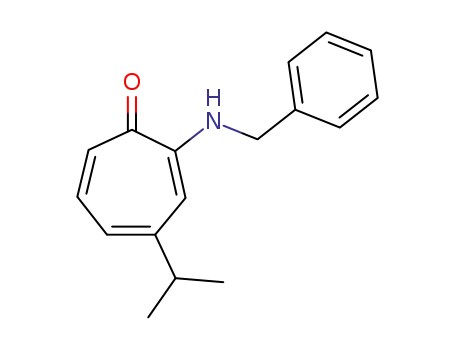
2-benzylamino-4-isopropyl-cycloheptatrienone
CAS:3081-61-6
CAS:85070-48-0
CAS:5994-61-6
CAS:10124-43-3